To the Editor:
Lymphomatoid papulosis is a rare chronic skin disorder characterized by recurrent, self-healing crops of papulonodular eruptions, often resembling cutaneous T-cell lymphoma.1 Oral bexarotene, a retinoid X receptor–selective retinoid, can be used to control the disease.2,3 Lichen planopilaris (LPP) is a type of cicatricial alopecia characterized by irreversible hair loss, perifollicular inflammation, and follicular hyperkeratosis, commonly affecting the scalp vertex in adults.4 We report a case of a patient with lymphomatoid papulosis who was treated with bexarotene and subsequently developed LPP. We also discuss a proposed mechanism by which bexarotene may have influenced the onset of LPP.
A 35-year-old woman who was previously healthy initially presented with recurrent pruritic papular eruptions on the flank, axillae, and groin of several months’ duration. The lesions appeared as 2-mm, flat-topped, violaceous papules. The patient had no known drug allergies, no medical or family history of skin disease, and was only taking 3000 mg/d of omega-3 fatty acids (fish oil). Histopathologic examination of a biopsy specimen from the inner thigh showed enlarged, atypical, dermal lymphocytes that were CD30+ (Figure 1). These findings were consistent with lymphomatoid papulosis. As she had undergone tubal ligation several years prior, she was prescribed oral bexarotene 300 mg once daily in addition to triamcinolone cream 0.1% twice daily, as needed. Symptoms were well controlled on this regimen.
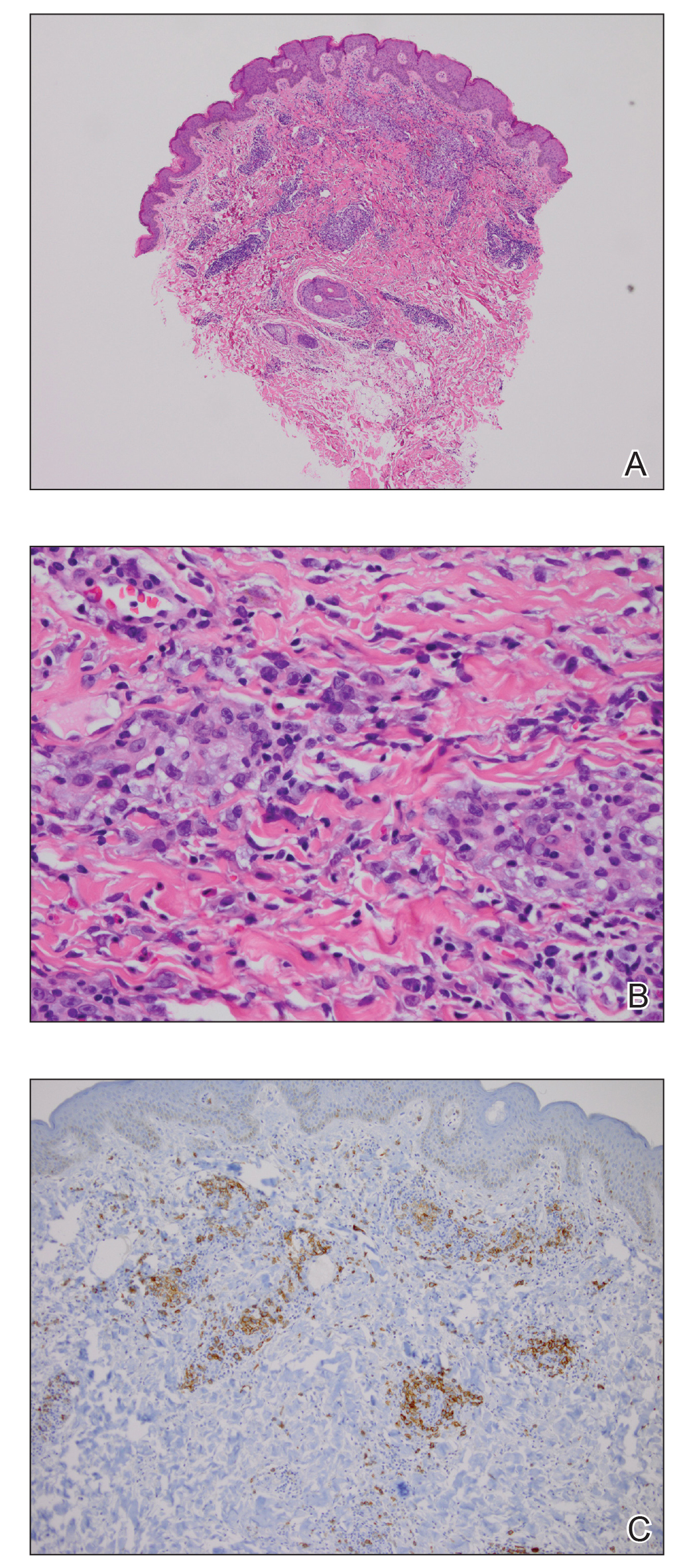
Figure 1. Histologic findings from a biopsy of the inner thigh. A, A superficial and deep, wedge-shaped lymphoid infiltrate was observed in the dermis (H&E, original magnification ×40). B, Highpower view showed a mixture of enlarged atypical lymphoid cells admixed with lymphocytes, histiocytes, and eosinophils (H&E, original magnification ×400). C, CD30 immunohistochemical stain highlighted an increase in CD30+ lymphoid cells arranged singly and in clusters (original magnification ×200).
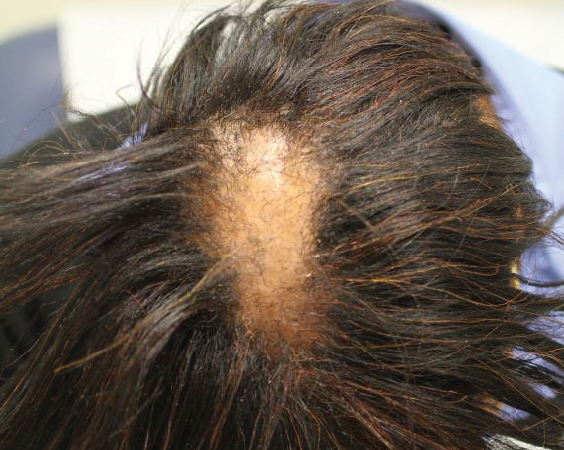
Six months later the patient returned, presenting with a new central patch of scarring alopecia on the vertex of the scalp (Figure 2). Adjacent to the area of hair loss were areas of prominent perifollicular scale that were slightly violaceous in color. Two 4-mm punch biopsies of the scalp showed dermal scarring with perifollicular lamellar fibrosis surrounded by a rim of lymphoplasmacytic inflammation (Figure 3). Sebaceous glands were found to be reduced in number. These findings were consistent with cicatricial alopecia, which was further classified as LPP in conjunction with the clinical findings. No CD30+ lymphocytes were identified in these specimens.
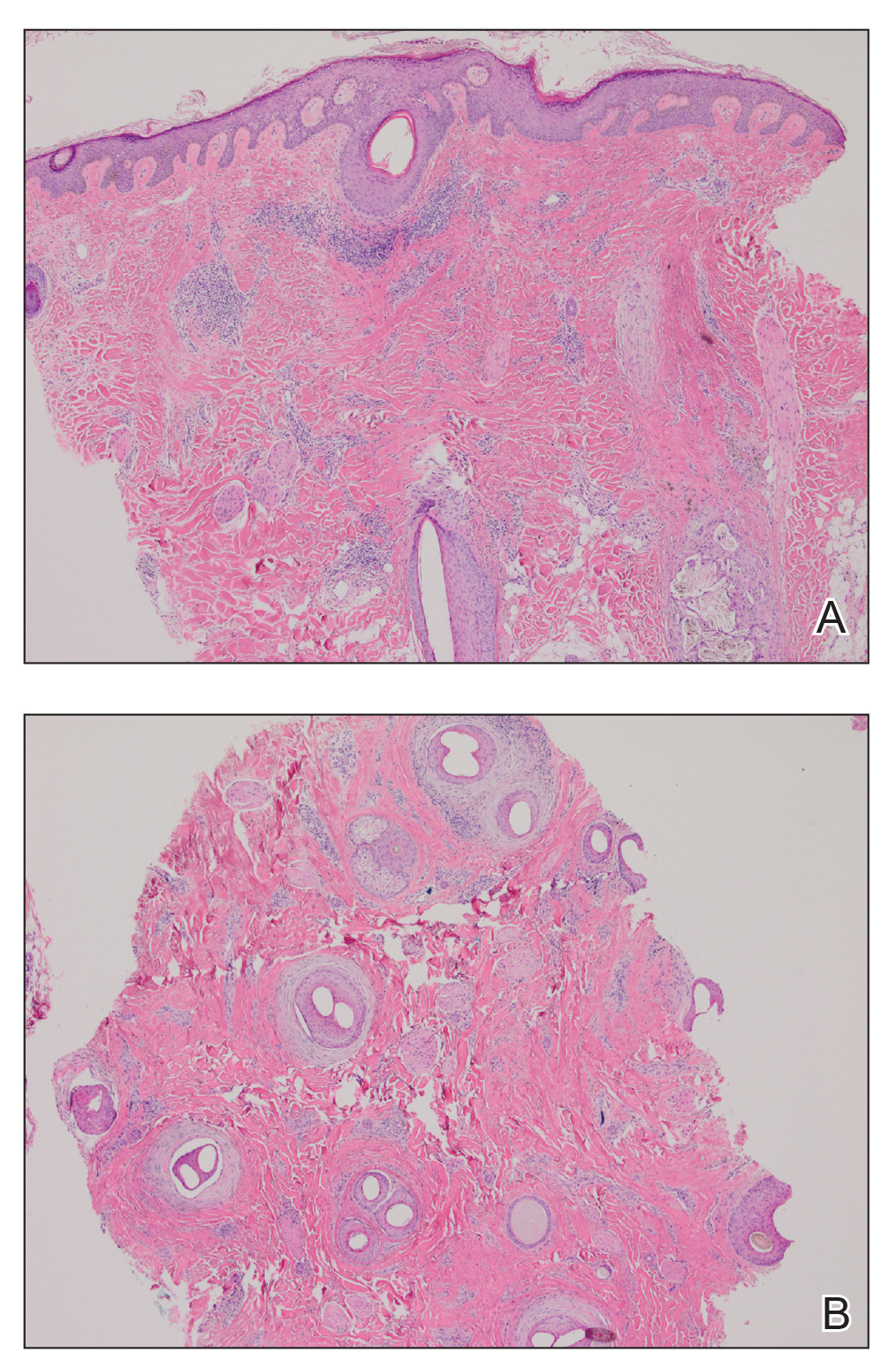
Figure 3. Histologic findings from a biopsy of the vertex of the scalp. A, Vertical section showed dermal fibrosis and perifollicular chronic inflammation (H&E, original magnification ×40). B, Horizontal section showed follicular dropout, perifollicular fibrosis, and perifollicular lichenoid inflammation (H&E, original magnification ×40).
Baseline fasting triglycerides were 123 mg/dL (desirable: <150 mg/dL; borderline: 150–199 mg/dL; high: ≥200 mg/dL) and were stable over the first 4 months on bexarotene. After 5 months of therapy, the triglycerides increased to a high of 255 mg/dL, which corresponded with the onset of LPP. She was treated for the hypertriglyceridemia with omega-3 fatty acids (fish oil), and subsequent triglyceride levels have normalized and been stable. Her alopecia has not progressed but is persistent. She continues to have central hypothyroidism due to bexarotene and is on levothyroxine. The lymphomatoid papulosis also remains stable with no signs of progression to cutaneous T-cell lymphoma.
Although the exact mechanism of LPP is not fully understood, studies have suggested that cellular lipid metabolism may be responsible for the inflammation of the pilosebaceous unit.4-11 Hyperlipidemia is the most common side effect of oral bexarotene, typically occurring within the first 2 to 4 weeks of treatment.3,12 Considering the insights into the role of lipid regulation on LPP pathogenesis, it is reasonable to suspect that the dyslipidemia caused by bexarotene may have triggered the onset of LPP in our patient. The patient’s lipid values mostly remained within reference range throughout the course of treatment, though she did have elevation of triglycerides around the onset of LPP. Dyslipidemia has been reported in patients with lichen planus but not in patients with LPP. One case-control study showed no dyslipidemia in patients with LPP, but the triglyceride levels were not tracked over time and patients had varying durations since onset of disease at presentation.9-11,13 In our case, we were fortunate to have this information, and it may suggest an interaction between lipid dysregulation and the development of LPP. It would be interesting to explore this further in a larger patient population and to evaluate if control of dyslipidemia reduces progression of disease as it appears to have done for our patient.