Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.
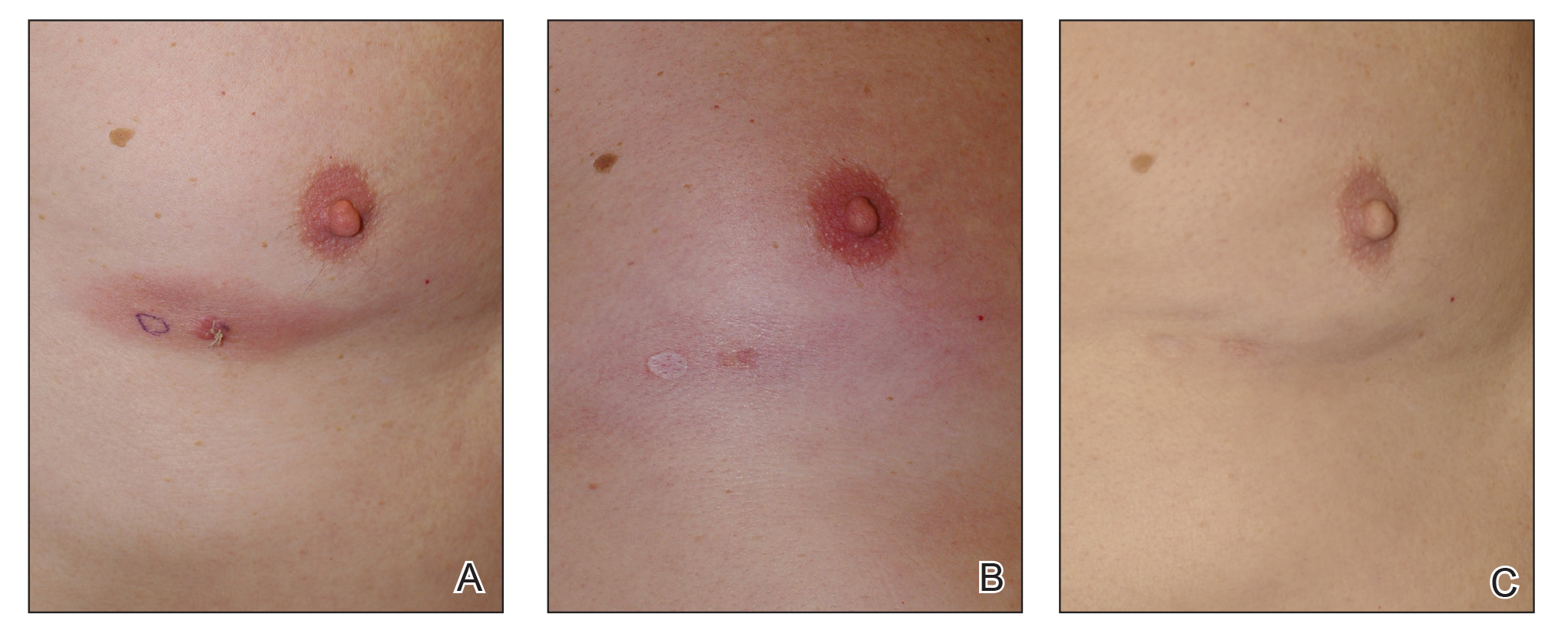
Figure 2. A, Erythematous mycosis fungoides patch on the left breast at baseline. B, Local skin hypopigmentation seen after 6 months of treatment with topical clobetasol propionate twice daily. C, Repigmentation of the skin was documented 1 year later after clobetasol propionate was tapered to 2 to 3 times weekly.
Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.

