The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission. The American National Standards Institute is a nonprofit group composed of laser manufacturers, government agencies, professional societies, educational institutions, and consumer and labor groups. It publishes voluntary safety standards and periodic updates (the series is labelled ANSI Z136) for the use of lasers in general (ANSI Z136.1) and for health care use in particular (ANSI Z136.3), including their use in dermatology. Laser hazard classifications also originate from ANSI. The standards of care established by ANSI guidelines are those by which health care providers are judged in health care litigation and are used by the other 3 organizations listed above. The Center for Devices and Radiological Health oversees laser manufacturers and their adherence to safety standards, determines laser hazard classifications such as ANSI, and requires manufacturers to affix a hazard class to the laser when manufactured. The Joint Commission is the accreditation body for health care programs and inspects hospitals and clinics for compliance with ANSI standards. Additionally, the American Society for Laser Medicine and Surgery, the American Academy of Dermatology, and the American Society for Dermatologic Surgery are professional organizations involved in laser operational safety training.3
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
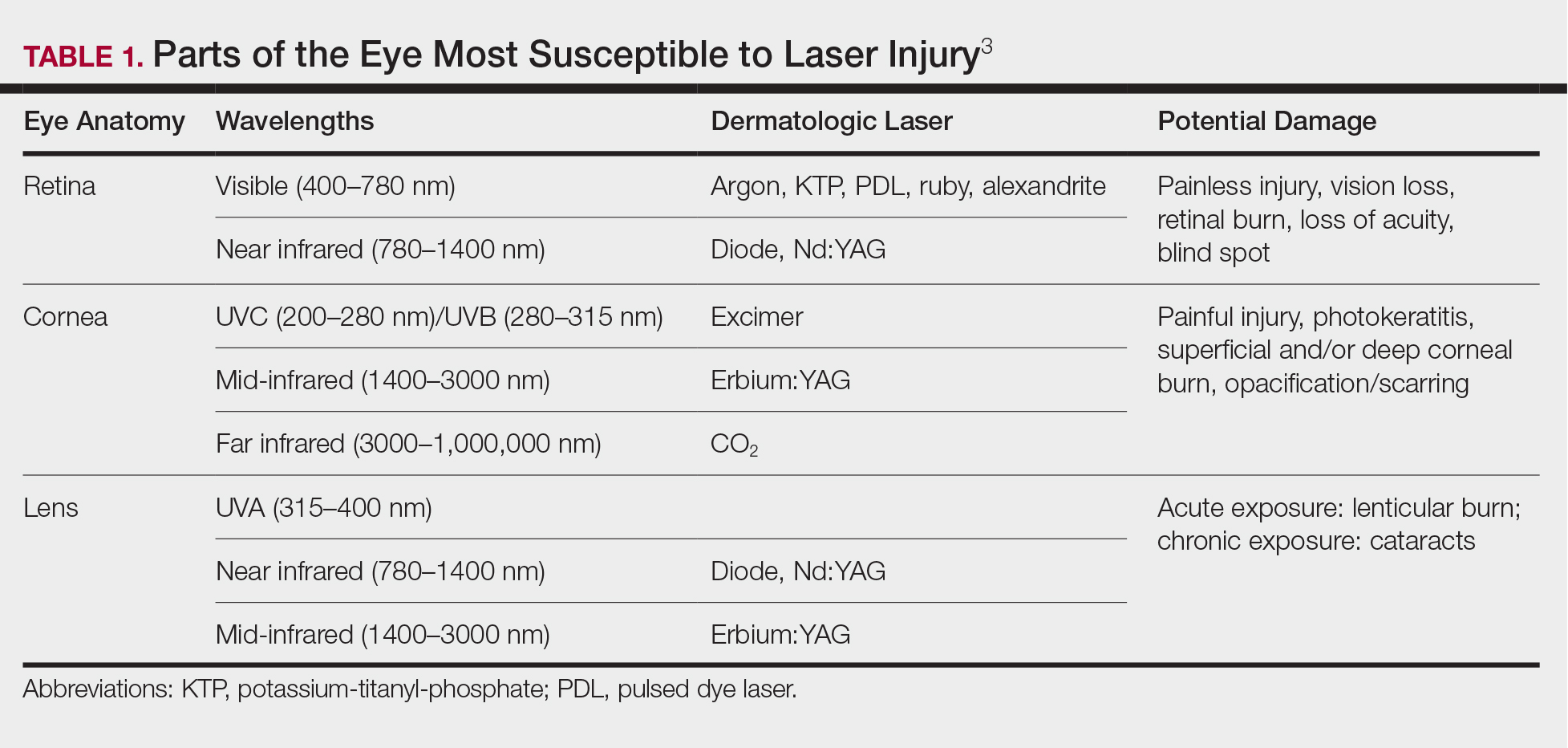
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3
The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.