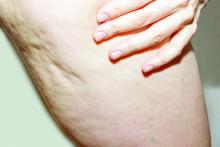
As described in a press release issued by Soliton, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin at a rate of up to 100 pulses per second to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples. The procedure takes 40-60 minutes to perform.
“This is a novel, noninvasive treatment for cellulite that appears to be safe, with little pain and little downtime,” Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said in an interview. “Further study and experience will determine the efficacy of this device and the optimization of its parameters going forward.”
In clinical trials that were part of the FDA’s 510(k) application process, patients underwent a single, noninvasive treatment that required no anesthesia and caused no unexpected or serious adverse events. The procedure also received strong patient satisfaction ratings, and clinical trial participants rated their average pain score as 2.4 out of 10.
Soliton plans to begin selling the device for both tattoo removal and cellulite treatment in the first half of 2021. “While the technology is broadly the same, the replaceable treatment cartridges [for tattoo removal and cellulite treatment] differ in significant ways,” Dr. Avram said.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.