Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
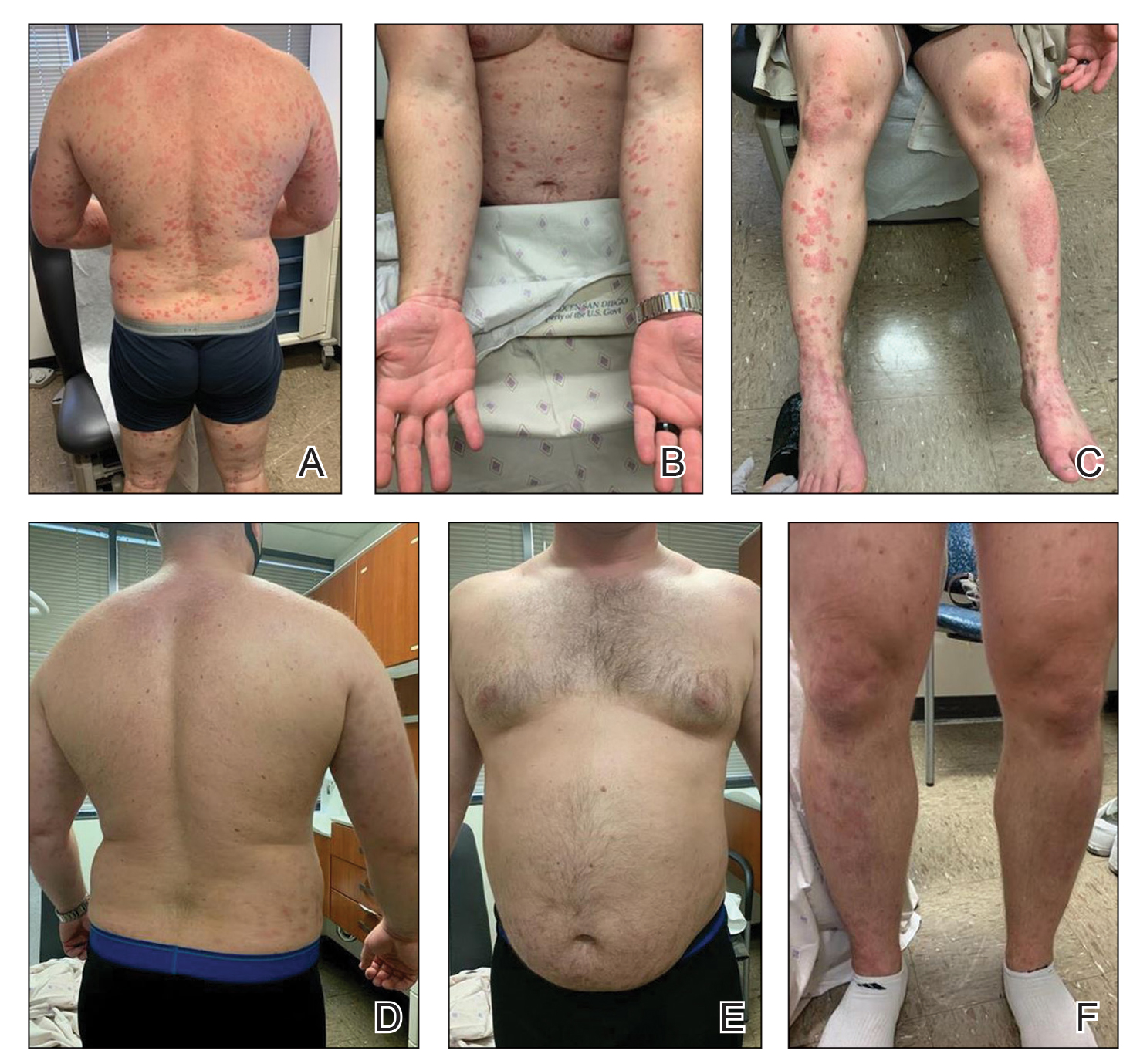
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.