CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
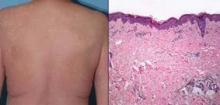 Courtesy Dr. James J. Harding
Courtesy Dr. James J. Harding
Left: A patient, previously treated with ipilimumab, developed a severe maculopapular rash within 8 days of starting vemurafenib. Right: A skin biopsy demonstrates a spongiotic, perivascular, and periadnexal dermatitis with subtle vasculopathic changes consistent with a drug hypersensitivity reaction.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.

