BARCELONA – When added to maximally tolerated statin therapy, the investigational PCSK9 inhibitor alirocumab resulted in a further 54% reduction in major cardiovascular events among high-cardiovascular-risk patients, based on a post-hoc analysis of a large randomized controlled Phase-3 trial.
The ODYSSEY LONG TERM trial is the largest and longest study of a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor to report results to date, with roughly 1,900 patient-years of double-blind exposure to alirocumab. And although the ongoing trial is primarily a safety study, it is also now the first PCSK9 trial to provide what everyone watching the development of this novel drug class has been eagerly awaiting: clinical outcomes data, albeit in this case from a post-hoc secondary analysis.
“This is the first trial with any of the PCSK9 inhibitors to suggest that there will be a further significant reduction in cardiovascular events when added on to maximized statin therapy,” Dr. Jennifer G. Robinson said in presenting interim results of ODYSSEY LONG TERM at the annual congress of the European Society of Cardiology.
“We’re on the right track in terms of trying to achieve further reduction in cardiovascular events through additional lipid lowering. But this is not the definitive evidence. We need the prospective outcomes trials to validate this data and also to establish the long-term safety of these drugs when added to the statins,” cautioned Dr. Robinson, professor of epidemiology and of medicine and director of the prevention intervention center at the University of Iowa, Iowa City.
Nonetheless, on the basis of the dramatic LDL-lowering and reassuring evidence of safety shown in ODYSSEY LONG TERM and the other double-blind phase III trials presented at the congress, Sanofi and Regeneron announced plans to file for U.S. and European Union marketing approval of alirocumab before the end of the year. The proposed indication will be for LDL lowering, which regulatory agencies have accepted as a surrogate endpoint for prevention of clinical events.
Meanwhile, the definitive ODYSSEY OUTCOMES trial is underway in 18,000 patients with acute coronary syndromes, with prospective evaluation of CV outcomes as its primary endpoint. The composite endpoint employed in the big OUTCOMES trial is identical to that used in the ODYSSEY LONG TERM post hoc analysis.
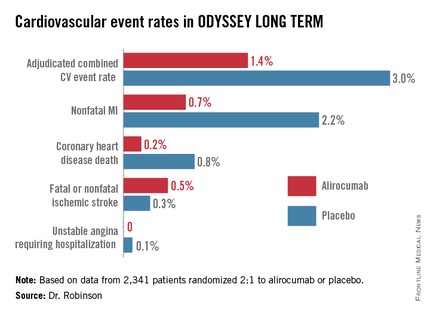
ODYSSEY LONG TERM includes 2,341 patients at high CV risk and an LDL level greater than 70 mg/dL despite maximally tolerated statin therapy. The patients fall into two categories: those with heterozygous familial hypercholesterolemia and others at very high risk because of known coronary heart disease. Participants were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection at home every 2 weeks or placebo in addition to their statin.
In the interim post-hoc efficacy analysis at 65 weeks, the combined rate of cardiac death, nonfatal MI, stroke, and unstable angina requiring hospitalization was 1.4% in the alirocumab arm compared to 3.0% in placebo-treated controls, for a highly significant 54% relative risk reduction (see graphic).
At 24 weeks, the alirocumab group showed a mean 62% reduction in LDL compared to placebo, a difference that remained constant at 52 weeks. The average LDL level at 52 weeks in the alirocumab group was 53 mg/dL, down from 123 mg/dL on active treatment at baseline; 79% of alirocumab-treated patients achieved an LDL below 70 mg/dL.
The incidence and types of adverse events in the alirocumab arm were essentially the same as with placebo, with no signal of problems in any domains, including neurocognitive function or allergic reactions.
ODYSSEY FH I and FH II
In a separate presentation, Dr. Michel Farnier reported on the alirocumab experience in 735 patients with heterozygous familial hypercholesterolemia in two phase III trials known as ODYSSEY FH I and FH II. At baseline, all were above their LDL goal despite maximally tolerated statin therapy, in two-thirds of cases with add-on ezetimibe. Participants were randomized 2:1 to add-on alirocumab at 75 mg every 2 weeks or to placebo.
The alirocumab-treated patients had 58% and 51% reductions in LDL, compared to actively treated controls at 24 weeks in the FH I and FH II trials. Of the alirocumab-treated patients, 72% and 81% achieved their prespecified LDL goal at 24 weeks, compared with 2% and 11% of controls.
“We have never before seen these kinds of percentages of patients with familial hypercholesterolemia reaching these LDL levels,” commented Dr. Farnier of Point Medical in Dijon, France.
ODYSSEY COMBO II
At the same hot-line clinical trials session, Dr. Christopher P. Cannon reported that alirocumab markedly outperformed ezetimibe as add-on therapy in the 720-patient, phase III, double-blind ODYSSEY COMBO II trial.