Antiviral Therapy for Chronic HCV
Prior to mid-2011, the standard of care for HCV treatment was the combination of pegylated interferon and ribavirin. From 2011 through 2013, direct-acting antiviral (DAA) regimens containing boceprevir and telaprevir in combination with pegylated interferon and ribavirin became standard of care for genotype 1 while
the standard of care remained pegylated interferon and ribavirin for genotypes other than genotype 1. Recent advances in HCV antiviral therapy offer higher cure rates and fewer adverse events (AEs) compared with peginterferon-containing treatment. The expected ease and tolerability of these all-oral combination regimens is anticipated to greatly increase the number of veterans with HCV who could be treated successfully.
Because of the poor tolerability, prolonged treatment durations, serious AEs, and relative or absolute contraindications to peginterferon-based therapy, many veterans were not previously candidates for treatment. Of the 174,302 veterans with chronic HCV in care in 2013, 39,388 (23%) had received at least 1 course of HCV antiviral treatment. This largely reflects the time when peginterferon-based therapy was the standard of care. Since the approval of boceprevir and telaprevir 5,732 veterans (5.8%) in care in 2013 had ever received boceprevir or telaprevir-based regimens.
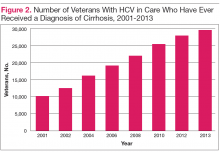
While recognizing that all veterans should be considered for HCV treatment, the urgency for treatment may be greater in those with advanced liver disease, because these patients are at the highest risk of developing decompensated cirrhosis or dying of liver-related disease. In 2013, there were 28,945 veterans in care that had advanced liver disease who might be considered potential HCV treatment candidates with an urgency to treat.
Duration of treatment and anticipated rates of treatment success with the all-oral regimens depend in part on a patient’s prior treatment status in addition to whether the patient has a diagnosis of advanced liver disease/cirrhosis. Regardless of HCV genotype, among all veterans approximately 85% are treatment-naïve and 15% are treatment-experienced. Advanced liver disease is present in 24% of treatment-naïve and 31% of treatment-experienced veterans with HCV genotype 1; 23% and 24% of veterans with HCV genotype 2, respectively; and 34% and 43% of veterans with HCV genotype 3, respectively.
Further understanding the population of veterans with HCV, including prior treatment status and stage of liver disease, is useful in identifying the target population for treatment. The VA uses these data to project treatment costs and assess capacity across the system in preparation for expected uptake of new regimens.
Sustained Virologic Response After HCV Antiviral Treatment
The goal of HCV antiviral therapy is to eradicate HCV and reduce the progression of liver disease and death from HCV infection. Successful antiviral treatment of HCV is determined by achieving a sustained virologic response (SVR) defined as an undetectable HCV viral load 12 weeks after the end of treatment. Of the 39,388 veterans in VA care in 2013 who have ever received antiviral therapy, SVR could be assessed in 32,815 veterans, and the overall SVR rate in this population was 42%. This SVR rate is similar to that observed in phase III trials of pegylated interferon-based regimens, where 42% to 46% of those infected with HCV genotype 1 achieved SVR. 7,8 Although most veterans with genotype 1 infection received boceprevir or telaprevir-based regimens in 2013 and achieved higher SVRs of 50% to 52%, the overwhelming majority of veterans in care in 2013 received prior treatment with only peginterferon and ribavirin. 9 Although SVR rates are expected to increase with newer all-oral HCV regimens, differences between clinical efficacy and real-world effectiveness will continue to be apparent,
and patient and provider expectations should be tempered accordingly.