Results
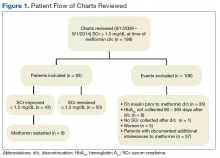
Of the 199 patients who had metformin discontinued because of elevated SCr (> 1.5 mg/dL), 106 were excluded for reasons listed in Figure 1; the other 93 met the study inclusion criteria and had their cases analyzed for change in glycemic control after discontinuation of metformin.
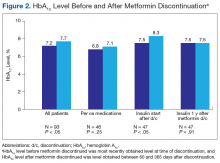
The mean age was 68.2 years and mean weight was 97.36 kg for the included patients. Four were African American, and 89 were white. At time of discontinuation of metformin, mean SCr level was 1.65 mg/dL, and mean eGFR was 43.25 mL/min/1.73 m2 (Table). Mean (SD) HbA1c level was 7.2% (1.1%) before discontinuation of metformin and 7.7% (1.5%) after discontinuation (P < .05) (Figure 2). Subgroup analysis of patients initiated on insulin after discontinuation of metformin (n = 47) revealed mean (SD) HbA1c levels of 7.5% (1.1%) before discontinuation and 8.3% (1.3%) after discontinuation (P < .05). One year or more after discontinuation of metformin in patients in whom insulin was initiated, mean HbA1c level decreased to the prediscontinuation (baseline) level of 7.5% (P = .91).
Twenty of these patients initiated on insulin had improved renal function and would have met the criteria for restarting metformin. After discontinuation of metformin, mean (SD) time to next recorded SCr level was 95.7 (89.9) days. Of the 93 study patients, 43 met the criterion for reinitiating metformin (rechecked SCr level, < 1.5 mg/dL), but in only 9 (21%) of these patients was metformin restarted.
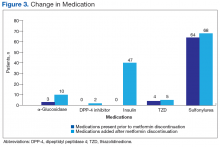
Medication changes made after discontinuation of metformin were assessed. Overall, 8% of the patients were started on α-glucosidase inhibitors, 4% on sulfonylureas, 2% on dipeptidyl peptidase 4 inhibitors, 1% on a thiazolidinedione, and 51% (47 patients) on insulin after discontinuation of metformin (Figure 3). Mean (SD) eGFR was 43.25 (7.3) mL/min/1.73 m2 when metformin was discontinued. Of the 93 patients at time of discontinuation of metformin, 37 (40%) had an eGFR over 45 mL/min/1.73 m2, 49 (53%) had an eGFR between 45 and 30 mL/min/1.73 m2, and 7 (7%) had an eGFR under 30 mL/min/1.73 m2. In addition, there were no cases of lactic acidosis among patients when metformin was initially discontinued.Discussion
Overall, a decline in glycemic control was found in patients who had metformin discontinued. This anticipated decline prompted clinicians to replace metformin with other oral medications as well as insulin. Despite DM medication regimen changes, mean HbA1c level increased significantly after discontinuation of metformin. The initial decline in glycemic control after starting insulin could be attributable either to clinician preference in insulin initiation—starting at lower doses to avoid hypoglycemia—or to a delay in initiating insulin, as opposed to initiating insulin at time of discontinuation of metformin. Subgroup analysis of the large number of patients who started on insulin after discontinuation of metformin (51%) revealed the same HbA1c levels before and 1 year after discontinuation. This finding is clinically relevant because many patients showed a decline in glycemic control for a year, despite initiation of insulin therapy.
In 43 (46%) of the 93 patients studied, SCr level improved to < 1.5 mg/dL after discontinuation of metformin. Of the patients in the subgroup started on insulin, 20 had improved renal function. This finding suggests that many of the patients who were initiated on insulin showed an improvement in renal function and potentially could have had metformin reinitiated. If these patients had continued or restarted metformin, insulin therapy may have been avoided or delayed. Overall, many opportunities to resume metformin were missed; only 9 of the 43 patients with improved SCr levels (< 1.5 mg/dL) on recheck were restarted on metformin. Many clinicians seemed hesitant to restart metformin even after kidney function improved. In addition, mean time to next recorded SCr level after discontinuation of metformin was 95.7 days. If SCr levels are more closely monitored after discontinuation of metformin, metformin possibly could be restarted sooner, leading to improved glycemic control and prevention of both microvascular and macrovascular complications.
In its 2012 update, the NKF suggested that it may be reasonable to consider using an eGFR cutoff when prescribing metformin. Clearance of metformin is reduced by 75% when eGFR is under 60 mL/min/1.73m2 but declines no further until eGFR is < 30 mL/min/1.73 m2.7 A systematic review of 65 articles found that, overall, levels of metformin remained in the therapeutic range, and lactate concentrations did not increase significantly in mild-to-moderate renal impairment (eGFR, 30-60 mL/min/1.73 m2).8 This finding corresponds to the updated 2016 FDA recommendations regarding use of metformin in mild-to-moderate renal impairment.
In the present study, patients who had metformin discontinued earlier, under strict package labeling, may have been able to continue metformin with use of eGFR under the revised labeling. Thirty-seven patients had an eGFR > 45 mL/min/1.73 m2 at the time of discontinuation of metformin, 49 had an eGFR between 45 and 30 mL/min/1.73 m2, and 7 had an eGFR < 30 mL/min/1.73 m2. Only 7 (8%) of the 93 patients would have had a contraindication to continuing metformin on the basis of current FDA recommendations. Forty-nine patients (53%) could have continued metformin if the benefit outweighed the risk, and 37 (39%) could have continued metformin given an eGFR > 45 mL/min/1.73 m2. The earlier labeling required initial discontinuation of metformin in these patients, but new FDA recommendations would allow more of them with mild-to-moderate renal impairment to benefit from treatment with metformin.