The American Heart Association, the American College of Cardiology, and the Obesity Society define overweight as a body mass index (BMI) of 25 to 29.9 and obesity as a BMI ≥ 30. Morbid obesity is defined as a BMI ≥ 35 or 40.2,3 Based on these BMI cutoffs, the Endocrine Society recommends diet and lifestyle as the foundation of weight management and pharmacotherapy for those with a BMI ≥ 30 without comorbidities. In patients with a BMI ≥ 27, weight management medications may be considered if a patient has comorbid hypertension, T2DM, dyslipidemia, metabolic syndrome, obstructive sleep apnea, or nonalcoholic fatty liver disease. Patients with BMI > 40 are eligible for weight loss surgery.4
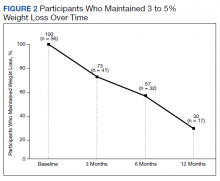
Lifestyle and dietary interventions are the foundation of current weight management guidelines from the Endocrine Society.4 At a minimum, guidelines recommended enrolling motivated patients in a high-intensity lifestyle intervention class of at least 14 sessions in the first 6 months to reach a goal weight loss of 5 to 10% from baseline and to maintain a reduction of 3 to 5% from baseline.3 Medications are recommended as an adjunct to lifestyle and dietary changes. Most weight management medications work in the brain to stimulate satiety signaling, which helps motivated patients adhere to their dietary interventions, assist those who have been unsuccessful in earlier weight loss attempts, and help maintain weight.3,4
Guidelines recommend 7 weight management medications, including orlistat (both prescription strength and over-the-counter), liraglutide, phentermine, phentermine/topiramate, lorcaserin, and naltrexone/bupropion. Using medications to assist with weight loss increases likelihood that patients will achieve 5 to 10% weight loss from baseline.5,6 Studies looking at long-term effects of these medications on weight loss have found improvements in blood pressure (BP), biomarkers for cardiovascular disease, and T2DM-related comorbidities.3,5,7
Positive effects on comorbidities have been found to be related to drug class and mechanism of action (MOA); those that also are approved for T2DM have demonstrated the most favorable cardiovascular effects.7 Other medications that work as stimulants or as modulators of serotonin pathways are associated with increased risks, prompting the US Food and Drug Administration (FDA) to remove some medications from the market.7,8 In January 2020, lorcaserin was taken off the market because of increased risk of cancer found in postmarketing surveillance.9 The benefit of weight loss must be weighed against the risk of medication use.
Monthly follow-up is recommended with weight management medications in the beginning to assess safety and efficacy; medications should be discontinued if weight loss is inadequate in the first 3 months.1,3,4 Limited studies have assessed the long-term use of weight management medications in a real-world setting. Medications are prescribed for weight management at Veteran Health Indiana (VHI) in outpatient clinics, including primary care, endocrinology, and gastrointestinal (GI) specialties. However, prescribing practices, outcomes, and adherence to guideline recommendations have not been studied. Data from this study will be used to better understand how VHI can serve its veterans through diet, lifestyle, and pharmacologic interventions.
Methods
We conducted a single-center, retrospective chart review for patients started on weight management medications at VHI. A patient list was generated based on prescription fills from June 1, 2017 to June 30, 2019. All data were obtained using the Computerized Patient Record System and patients were not contacted. This study was approved by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.