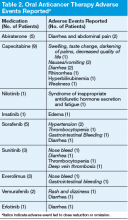
Adherence assessments using pill counts and medication calendars demonstrated that 6% (121/2,037) of doses that were dispensed were not taken. Overall adherence rate was 94%. The average patient adherence rate was 93.2%. Adverse events contributed to 62.8% of doses omitted (Table 2). Some AEs (eg, nausea, vomiting, and hypertension) were deemed preventable or modifiable with better symptom management. However, the majority of AEs that led to dose omission were not preventable.
Ten patients had their treatments discontinued midcycle, leading to 24.7% of missed doses. Adverse events led to 70% of discontinuation, whereas 20% resulted from disease progression. In both cases of disease progression, the patient was given a 30-day supply before the restaging scan, and in both cases this led to oral anticancer therapy waste. An additional 12.3% of doses were omitted due to hospitalization of patients.
Over a 6-month period, an estimated $32,314 was saved under the 14-day dosing pilot. This number was reached by subtracting the number of pills actually dispensed under pilot protocol from the number of pills that would have been dispensed under old dispensing standards (usually 28- to 30-day supply), then multiplying the difference by the cost per pill.
The results of this study were presented to the Pharmacy and Therapeutics Committee and led to the approval to continue with the 14-day dispensing protocol at VAPHS in March 2012. In addition, the pilot served as the backbone for the VHA Guidance on Oral Chemotherapy Dispensing and Monitoring. 10 As part of the guidance, a monitoring guide for all the FDA-approved oral anticancer therapies is maintained and available for all VA practitioners to access on the PBM website under the Clinical Guidance subheading.
Current Practice
From the time the original pilot was conducted, the number of available oral anticancer therapies has increased along with the patient volume. Due to these factors and the lack of a dedicated outpatient oncology clinical pharmacist, oncology nurses in the outpatient clinic now direct the education, dispensing, and monitoring of patients on oral chemotherapy.
Treatment Plan
An oral anticancer treatment plan is developed by the oncology physician and entered in the EMR as a progress note titled Treatment Plan. The treatment plan includes, disease, stage, curative vs palliative intent, premedications, oral anticancer medication, dose, route and frequency, cycle length and number of cycles, baseline and continuous monitoring parameters, follow-up with provider, and staging follow-up. Once the oncology clinical pharmacist approves a treatment plan, the oncology nursing staff ensures that all the prechemotherapy laboratory tests are ordered and helps arrange any additional tests needed (echocardiogram, electrocardiogram, etc). After all the prechemotherapy testing is complete, the oncology nurse phones the patientto schedule a date for chemotherapy education and to pick up the first 14-day supply.
Initial Visit
The oncology nurse meets with each patient receiving oral anticancer therapy and provides them with an oncology clinic information packet, which includes chemotherapy education, a medication sheet, questions and answers about chemotherapy, common AEs and ways to manage them, as well as tips for meeting with the nurse and physician. The oncology nurse then reviews the oral anticancer treatment the patient is to receive, including how to administer the medication and timing, whether to take with or without food, common AEs, storage, safe handling, contact name if a toxicity arises, and importance of adherence.