IMMUNOTHERAPY
CTLA-4 AND PD-1 IMMUNE CHECKPOINT INHIBITORS
The novel success of immunotherapy in recent decades is largely attributable to improved understanding of adaptive immune physiology, specifically T-cell activation and regulation. T-cell activation requires 2 independent signaling events: it is initiated upon recognition of the antigen-MHC class II-receptor complex on antigen-presenting cells (APC), and requires a secondary co-stimulatory interaction of CD80/CD86 (B7.1/B7.2) on APCs and CD28 molecule on T-cells; without this second event, T-cells enter an anergic state.30–32 Upon successful signaling and co-stimulation, newly activated T-cells upregulate CTLA-4, which can bind to B7 molecules with a nearly 100-fold greater affinity than CD28.33,34 Unlike CD28, CTLA-4 engagement negatively regulates T-cell activation. The opposing signals produced by CD28 and CTLA-4 are integrated by the T-cell to determine eventual response to activation, and provide a means by which T-cell activation is homeostatically regulated to prevent exaggerated physiologic immune responses.35 It was hypothesized that CTLA-4 blockade would permit T-cell activation, which is thwarted in the tumor microenvironment by tumor-mediated CTLA-4 engagement, thereby unleashing an anti-tumor immune response.36
PD-1 is a member of the CD28 and CTLA-4 immunoglobulin super family and, similar to CTLA-4, binds activated T-cells. PD-1 has 2 ligands on activated T-cells: PD-L1 and PD-L2.37 PD-L1 is constitutively expressed by a variety of immune and non-immune cells, particularly in inflammatory environments including tumor microenvironments, in response to the release of inflammatory cytokines such as interferon (IFN)-γ.37,38 Conversely, PD-L2 is only minimally expressed constitutively, although its expression on immune and non-immune cells can be induced by similar cues from inflammatory microenvironments. PD-L1 and PD-L2 cross-compete for binding to PD-1, with PD-L2 exhibiting 2- to 6-fold greater relative affinity than PD-L1.39 PD-L1/PD-1 binding results in phosphorylation of 2 tyrosinases in the intracellular portion of PD-1, which contains immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM). PD-1 ITSM subsequently recruits either of 2 SH2-domain–containing protein tyrosine phosphatases: SHP-1 and SHP-2. SHP-2 signaling suppresses PI3K/Akt activation, down-regulates Bcl-xL, and suppresses expression of multiple transcription factors that mediate T-cell effector function including GATA-3, Eomes, and T-bet.40–42 The net effect of PD-L1/PD-1 engagement is to suppress T-cell proliferation, cytokine production, cytolytic function, and survival. Unlike CTLA-4, which primarily affects the priming phase of naive T-cell activation, PD-1 chiefly regulates the effector phase of T-cell function. Furthermore, because PD-L1/PD-L2 expression is limited to inflammatory microenvironments, the effects of PD-1 are less generalized than those of CTLA-4.
SINGLE AGENT ACTIVITY OF CTLA-4 AND PD-1 INHIBITORS
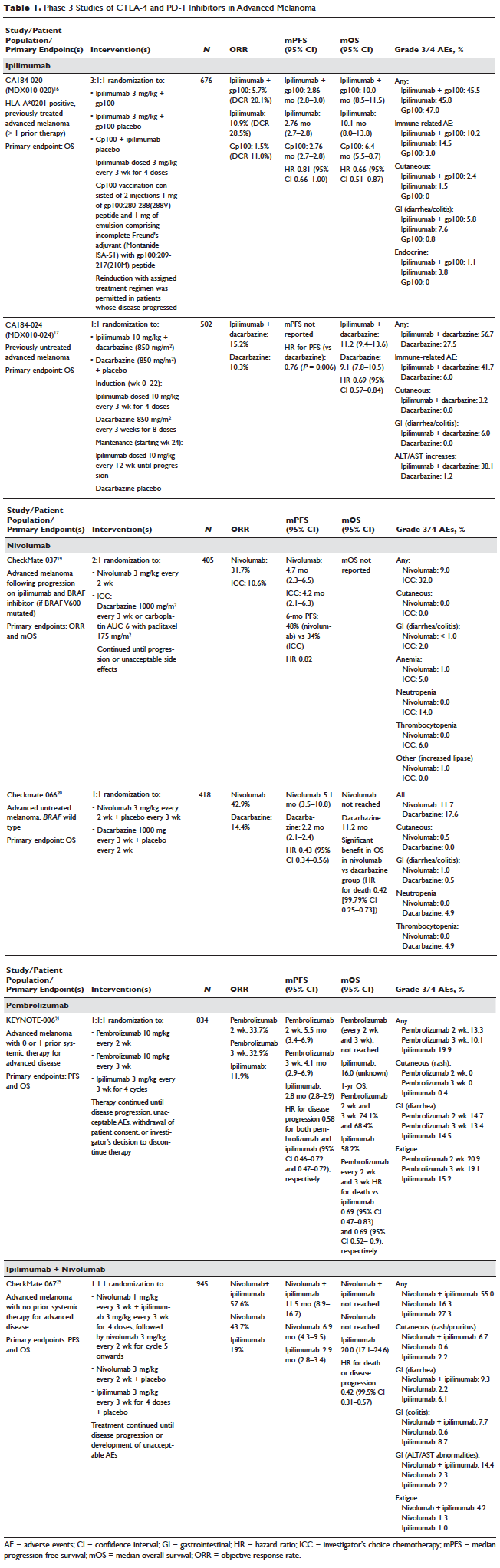
Ipilimumab (MDX-010) is a human IgG1 monoclonal antibody shown to inhibit CTLA-4.43 Early studies tested different formulations (transfectoma-derived and hybridoma-derived), doses, and schedules of ipilimumab primarily in patients with advanced refractory melanoma.44–46 Although responses were infrequent, responding patients experienced durable remissions at 1- and 2-year time points. Notably, in a foreshadowing of changes to response criteria used to evaluate these agents, several treated patients who initially had radiographically stable disease upon completion of therapy subsequently experienced a gradual decline in tumor burden.
Ipilimumab was subsequently evaluated in 2 phase 3 trials. The first study (MDX010-020/CA184-020), which involved 676 HLA-A*0201–positive patients with advanced melanoma, compared ipilimumab 3 mg/kg every 3 weeks for 4 doses either singly or in combination with gp100 vaccine with a gp100-only control arm.16 Ipilimumab administration resulted in objective responses in 11% of patients and improved progression-free and overall survival compared to gp100 alone. Of note, ipilimumab monotherapy was superior to ipilimumab/gp100 combination, possibly related to timing of vaccine in relation to ipilimumab. A confirmatory study (CA184-024) compared a higher dose of ipilimumab (10 mg/kg) in combination with dacarbazine to dacarbazine monotherapy in previously untreated melanoma and was positive.17 Given the lack of augmented efficacy with the higher (10 mg/kg) dose, ipilimumab received regulatory approval in 2011 for the treatment of melanoma at the lower dose: 3 mg/kg administered every 3 weeks for 4 doses (Table 1). Survival data was strikingly similar to patterns observed in prior phase 2 studies, with survival curves plateauing after 2 years at 23.5% to 28.5% of treated patients. Pooled survival data from prospective and retrospective studies of ipilimumab corroborate the plateau of 22% (26% treated; 20% untreated) reached at year 3 regardless of prior therapy or ipilimumab dose, underscoring the durability of long-term survival in ipilimumab-treated patients.47 Ipilimumab administration resulted in an unusual spectrum of toxicities including diarrhea, rash, hepatitis, and hypophysitis (termed immune-related adverse events, or irAEs) in up to a third of patients.