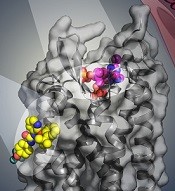
BPTU (yellow) bind to P2Y1R
Image courtesy of
Yekaterina Kadyshevskaya
Researchers say they have identified 2 disparate ligand-binding sites in the human P2Y1 receptor (P2Y1R), which plays a critical role in thrombosis.
Their research has provided a detailed molecular map of P2Y1R, a G protein-coupled receptor (GPCR), in complex with a nucleotide antagonist MRS2500 and a non-nucleotide antagonist BPTU.
The researchers believe their findings, published in Nature, could aid the development of new antithrombotic drugs.
Beili Wu, PhD, of the Shanghai Institute of Materia Medica in China, and her colleagues noted that the human purinergic receptors P2Y1R and P2Y12R play a major physiological role in adenosine 5′-diphosphate (ADP)-mediated platelet aggregation, an important component of thrombosis.
Although most of the available antithrombotic drugs act on P2Y12R, research has suggested that P2Y1R may be a promising antithrombotic drug target. In addition, P2Y1R inhibitors may offer a safety advantage over P2Y12R inhibitors by reducing the risk of bleeding. However, efforts to develop new drugs have been impeded by poor understanding of receptor-ligand interaction.
“The P2Y1R structures [we mapped] help us understand how this receptor and different types of experimental drugs interact at the molecular level and could enable further exploration to design new and safer antithrombotic drugs with reduced adverse effects,” Dr Wu said.
She and her colleagues found that the nucleotide ligand MRS2500 recognizes a binding site within the transmembrane bundle of P2Y1R. And it is different in shape and location from the nucleotide-binding site in P2Y12R.
“It is amazing to observe that 2 GPCRs recognize the same ligand in such different ways,” Dr Wu said. “The finding highlights the diversity of signal recognition mechanisms in GPCRs, and this is of great value to drug design for each receptor with high selectivity.”
The researchers also found that, instead of interacting within the transmembrane bundle, the non-nucleotide ligand BPTU binds to a pocket on the outer interface of P2Y1R embedded in the cell membrane.
This is the first structurally characterized, selective, and high-affinity GPCR ligand located entirely outside of the helical bundle, and it represents a new paradigm in ligand binding to alter signaling in GPCRs, according to the researchers.
The team believes this new understanding of the P2Y1R structure provides opportunities to broaden the scope of future GPCR drug discovery to target novel sites outside of the conventional GPCR ligand-binding pocket, which may improve drug selectivity and reduce side effects.


