The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved objective response rates (ORRs), progression-free survival (PFS), and overall survival (OS) for patients with metastatic melanoma. This article reviews current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy. The selection of first-line therapy for metastatic melanoma is reviewed in a separate article.
Case Presentation
A 62-year-old man was diagnosed with stage IIA melanoma after undergoing wide local excision of a right scalp lesion (final staging was consistent with pT3aN0M0). After 3.5 years of follow-up, he developed symptoms of vertigo, diplopia, and recurrent falls prompting medical attention. Magnetic resonance imaging (MRI) brain revealed multiple supratentorial and infratentorial lesions concerning for intracranial metastases and computed tomography (CT) chest/abdomen/pelvis revealed a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He was treated with intravenous dexamethasone and further evaluation with an endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass revealed metastatic melanoma. The patient underwent whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy. Testing showed the melanoma was positive for a BRAF V600K mutation. He was started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially did well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass.
After 3 months of therapy, surveillance PET-CT notes increasing size and FDG avidity of the right lower lobe mass. MRI brain reveals resolution of several previously noted metastases, but with interval development of a new left frontal lobe mass concerning for progressive disease.
What is the general approach to treatment of metastatic melanoma after progression on first-line therapy?
Based on the current evidence, there is no definitive algorithm for the treatment of metastatic melanoma after progression on first-line therapy. Enrollment in clinical trials is encouraged to further elucidate the best sequencing of treatment. The current practice is to typically switch class of agents after progression on front-line therapy to either immunotherapy that has not yet been tried or to molecularly targeted therapy in patients harboring a BRAF V600 mutation. After further progression of disease, retreatment with a previously received agent is possible, and this may be combined with investigational therapies.
Immune Checkpoint Inhibitors in Progressive Disease
The 2 major populations of patients to consider are those with BRAF wild-type melanomas who progress on first-line immunotherapy and those with BRAF V600 mutation–positive melanoma who progress on molecularly targeted therapy with BRAF and MEK inhibitors. There is relatively limited data on the efficacy of immune checkpoint inhibition after progression on anti-programmed cell death 1 (PD-1) monotherapy. A small retrospective study of patients who progressed on anti-PD-1 monotherapy were treated with ipilimumab, with a 10% ORR and another 8% having stable disease for more than 6 months; however, 35% of patients experienced grade 3 to 5 immune-related adverse events.1 The only prospective data supports the efficacy of anti-PD-1 therapy after progression on ipilimumab, as supported by the CheckMate 037 trial (nivolumab versus chemotherapy)2 and KEYNOTE-002 trial (pembrolizumab versus chemotherapy)3,4; however, this is no longer applicable as ipilimumab is no longer given in the first-line setting and has been replaced by anti-PD-1 monotherapy or combination immunotherapy.
Another interesting facet of PD-1 monotherapy is the idea of treatment beyond progression. The concept of pseudoprogression—whereby patients receiving PD-1 inhibitors initially meet Response Evaluation Criteria in Solid Tumors (RECIST) criteria for progression, but then later go on to demonstrate significant decreases in tumor burden on subsequent imaging studies—has been described in melanoma patients receiving such immunotherapies. It is thought that pseudoprogression occurs due to either an initial delay in anti-tumor response to the immunotherapy or from the measured target lesion appearing larger due to surrounding immune/inflammatory infiltrate. In an analysis of individual patient data pooled from 8 multicenter clinical trials, 19% of patients were treated beyond initially documented RECIST progression and had subsequent imaging to evaluate the tumor burden; in these patients, the same target lesion later met RECIST criteria for response, with a greater than 30% reduction in tumor size. Furthermore, of the evaluable cohort, the median OS in patients who did receive treatment beyond progression was 24.4 months compared to 11.2 months in those who did not receive treatment beyond progression.5 While further randomized studies are warranted to characterize the potential benefit, the existing data suggests that selected patients who are doing well clinically despite evidence of radiographic progressive disease may benefit from continued treatment with PD-1 inhibitors.
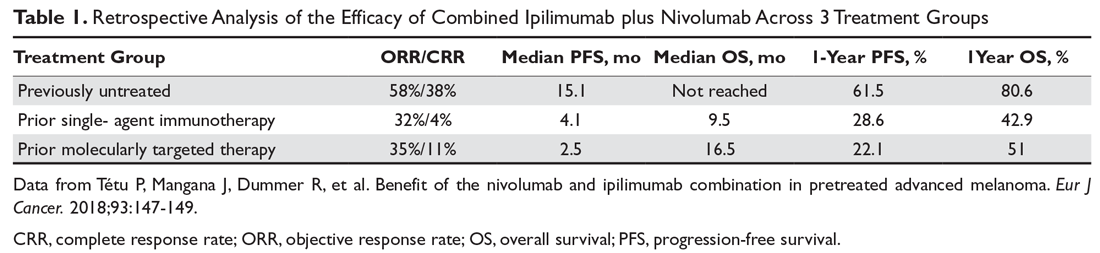
Combination immunotherapy with both PD-1 and CTLA-4 blockade has been studied retrospectively in the second-line setting. A retrospective analysis of patients who had progressive disease on PD-1 inhibitor monotherapy compared the outcomes of patients who received just ipilimumab to those of patients who received both ipilimumab and nivolumab. The ORR (16% ipilimumab vs 21% combination group) and 1-year OS (54% vs 55%) were similar in both groups,6 and this demonstrated significantly less efficacy with combination therapy when compared to use in the first-line setting, albeit in a separate prospective trial.7 A multicenter, retrospective study by Tétu and colleagues compared outcomes with ipilimumab plus nivolumab across 3 groups that included previously untreated patients, patients who had progressed on single-agent immunotherapy, and patients who had progressed on prior molecularly targeted therapy.8 Despite clearly inferior efficacy in previously treated patients, the results support combination immunotherapy as a viable treatment option in the second-line setting. Outcomes are reported in Table 1 below. Of note, there is an ongoing phase 2 trial to assess the use of combined PD-1 and CTLA-4 inhibitors versus CTLA-4 inhibition alone after progression on first-line PD-1 inhibitor monotherapy (NCT03033576).