It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
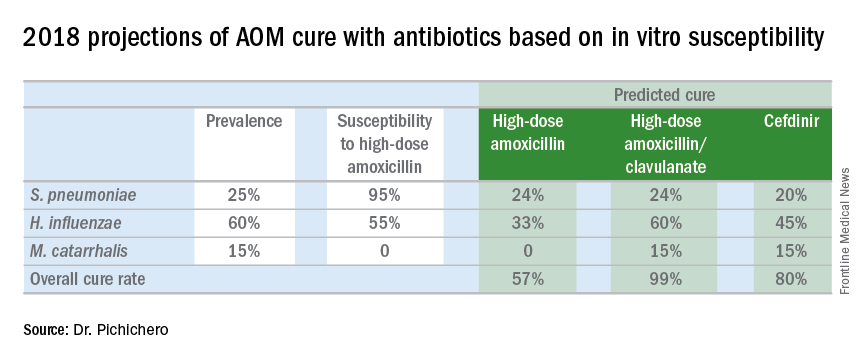
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains.
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He has no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.