Inpatient Screening
To improve screening of hospitalized patients with CF not known to have CFRD, 4 standardized order sets detailing blood glucose testing schedules were developed in our electronic medical record as listed below. Our team educated physicians and nurses on the standardized order sets as well as patient families about the additional testing needed during hospitalization. An endocrine nurse practitioner conducted daily rounds on weekdays. All glucose results were verified by the laboratory. If a patient was identified to have a fasting blood glucose ≥ 125 mg/dL or 2-hour postprandial ≥ 200 mg/dL, the endocrine service was notified, and additional glucose testing was ordered. If the patient’s glucose levels persisted at high levels, meeting the criteria for a diagnosis of CFRD, further education was provided for the family and follow-up care was arranged.
Order Sets
1) Not receiving G-tube feedings
Day 1: 2-hour postprandial
Day 2: Fasting
2) Receiving G-tube feedings
Day 1: 2-hour postprandial, 2-hours after start of
G-tube feeds and at end of G-tube feeds
3) Receiving low-dose steroid therapy (IV methylprednisolone up to 4 mg/kg per day, administered every 6 hours and oral prednisolone 1 mg/kg twice per day for 5 days) [12]
Day 1: 2-hour postprandial
Day 2: Fasting and 2-hour postprandial
Day 3: 2-hour postprandial
4) Receiving high-dose steroid therapy per the allergic bronchopulmonary aspergillosis protocol (IV methylprednisolone 10–15 mg/kg once per day for 3 days) [12]
Days 1-4: Point of care prior to every meal, at bedtime, and at 0200 hours
Analysis
All patients with CF who are seen at our clinic were included in this project. Approximately 96% of our patients have consented to be part of the CFF supported patient registry, PortCF [13]. Data are entered by the CF clinic nurse coordinator for these patients to document clinic visits, hospital admissions, medications, lab values, and other parameters. We retrieved data from PortCF documenting the number of patients eligible for the OGTT and the number of patients diagnosed with CFRD. Individual medical records were cross-referenced with these results and also reviewed for patients who do not participate in PortCF. We retrieved data reports from our hospital informatics department to identify the number of patients with CF who were hospitalized each year and to obtain all glucose levels obtained during hospitalization. From these data, we calculated screening rates and the annual number of patients diagnosed with CFRD. We used the Cochran’s Q test to compare the differences in frequency of screening across years.
Results
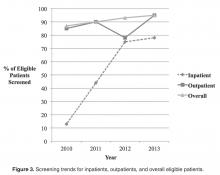
Screening of outpatients with OGTT improved from 84.6% in 2010 to 89.6% in 2011, decreased to 76.9% in 2012, and improved to 94.9% in 2013 ( Figure 3 ). At the conclusion of 2013, all patients who were previously administered the lower than recommended oral glucose dose were screened again.The newly implemented inpatient protocol, formally instituted in the third quarter of 2011, yielded a 43.8% screening rate in 2011 compared to a rate of 13.3% in 2010. Inpatient screening rates improved to 62.5% in 2012, and 77.8% in 2013 ( Figures 3 and 4 ).