From the University of California San Francisco, Department of Psychiatry, Weill Institute for Neurosciences, San Francisco, CA.
Abstract
- Objective: To review screening for metabolic syndrome in people with severe mental illness (SMI).
- Methods: Review of the literature.
- Results: Despite evidence-based metabolic screening guidelines, rates of metabolic screening remain low among people with SMI. Barriers to screening exist at the individual, organizational, and systems levels. Interventions to address these barriers range from point-of-care tools to systems-level reorganization towards population-based care.
- Conclusion: Greater systems-level interventions, particularly those that improve collaboration between mental health and primary care, are needed to improve metabolic monitoring and identify cardiovascular disease risk among people with SMI.
Key words: metabolic monitoring; severe mental illness; metabolic syndrome; integrated care.
People with severe mental illness (SMI) have a life expectancy 10 to 20 years shorter than the general population, and cardiometabolic risk factors contribute significantly to the increased morbidity and mortality seen in this population. To address this health disparity, metabolic monitoring guidelines have been proposed as a mechanism to identify metabolic risk factors. This paper aims to discuss metabolic syndrome and its risk factors, describe metabolic monitoring including current rates and barriers to screening, and identify interventions that may improve rates of screening for metabolic syndrome among people with SMI.
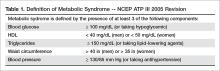
Metabolic syndrome has been conceptualized as a state of chronic low-grade inflammation and hypercoagulation associated with hypertension, dyslipidemia, glucose intolerance, insulin resistance, and visceral adiposity [1]. Per the modified National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) guidelines, metabolic syndrome is defined as the presence of 3 of the following 5 parameters: (1) blood glucose > 100 mg/dL (or a person is taking a hypoglycemic medication), (2) high density lipoprotein (HDL) < 40 mg/dL in men or < 50 mg/dL in women, (3) triglycerides > 150 mg/dL (or taking a lipid lowering agent), (4) waist circumference > 40 inches in men or > 35 inches in women, and/or (5) blood pressure > 130/85 mm Hg (or taking an antihypertensive medication) [2,3] (Table 1).
Metabolic syndrome is associated with an increased risk of diabetes mellitus, cardiovascular disease (including myocardial infarction and cerebrovascular accident), and all-cause mortality [3]. Other systemic effects related to metabolic syndrome include renal, hepatic, and skin manifestations such as chronic kidney disease, non-alcoholic steatohepatitis, and obstructive sleep apnea [1].
Epidemiology and Risk Factors
An estimated 34% of people in the United States meet criteria for metabolic syndrome, with worldwide estimates ranging widely from less than 10% to 84%. People with SMI (eg, bipolar disorder, schizoaffective disorder, schizophrenia) are at even greater risk of developing metabolic syndrome than the general population [4,5]. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated metabolic syndrome rates of 40.9% and 51.6% in men and women with a diagnosis of schizophrenia, respectively [6]. In a systematic review of bipolar disorder and metabolic syndrome, people with bipolar disorder showed higher rates of hypertriglyceridemia and hyperglycemia than controls [5].
People with SMI have been found to have significantly increased morbidity and mortality as compared to people without an SMI diagnosis, much of which has been attributed to increased cardiometabolic risk related to multiple factors [7]. Among adults with schizophrenia receiving Medicaid, Olfson et al found diabetes mellitus, ischemic heart disease, nonischemic heart disease, and cerebrovascular accident to be among the top 10 causes of death [7]. The mortality rate for people with SMI is estimated to be 2 to 3 times higher than the general population, and the life expectancy for people with SMI is estimated to be 10 to 20 years shorter than the general population [8–10]. Contributors to this disparity include modifiable health-related behaviors, social determinants of health, and iatrogenic sequelae of prescribed medications. Behavioral factors include poor nutrition, food insecurity, sedentary lifestyle, and smoking; side effects of commonly prescribed psychotropic medications, most notably atypical antipsychotics and mood stabilizers, also contribute to this disparity [7,11].
Both first- and second-generation antipsychotics have been shown to be associated with metabolic sequelae, including weight gain, elevated blood glucose, and insulin resistance [12–14]. Among psychotropic medications, the atypical or second-generation antipsychotics (SGAs) are a class of medications known to have significant metabolic side effects [15,16]. Studies comparing the metabolic consequences of individual SGAs have found significant variation within the class. Clozapine, olanzapine, quetiapine, and risperidone show significant likelihood of weight gain, hyperlipidemia, and hyperglycemia as well as other metabolic consequences [17]. Aripiprazole, lurasidone, and ziprasidone have shown little to no risk of metabolic sequelae [17].
Metabolic side effects of SGAs have been demonstrated in children, adolescents, and adults. There is evidence that adolescents may be particularly sensitive to these sequelae. Galling and colleagues found that adolescents treated with antipsychotics were at greater risk of developing type 2 diabetes mellitus as compared to both healthy controls and controls with psychiatric illness [18]. Kryzhanovskaya et al, looking at metabolic parameters associated with olanzapine use in adolescents and adults, found that both adolescents and adults showed metabolic sequelae and that adolescents had larger changes in weight gain and lipids compared with adults [19].
The mechanism of SGA impact on metabolic parameters remains incompletely understood, though is thought to be multifactorial, mediated primarily through weight gain with increased adiposity. SGA histamine (H1) receptor binding affinity is implicated in weight gain [20] and 5HT2C antagonism may also lead to an increase in appetite [21]. Other proposed mechanisms include changes in appetite through leptin resistance or decreased sensitivity to leptin, the hormone that mediates satiety. Zhang and colleagues found an increase in leptin levels in patients with schizophrenia prescribed antipsychotics, suggesting leptin dysregulation [21]. Additional studies suggest metabolic disturbances independent of weight gain including direct effects of SGAs on glucose and lipid metabolism [22].
If a person experiences a weight gain of 5% after starting an SGA, it is recommended that the dose be decreased or that they be switched to another psychotropic medication with lower likelihood of metabolic consequences [23]. The effectiveness of switching antipsychotic medications to one with lower metabolic risk to improve weight and lipids has been previously demonstrated [24]. If a patient develops diabetes in the context of an antipsychotic prescription, it is also recommended that the medication be switched to an antipsychotic with less risk of hyperglycemia, and if not possible, to target additional risk factors including weight, poor nutrition, and sedentary lifestyle [25]. The decision to switch medications or decrease dosage is often weighed against the psychiatric stability of the person and their overall response to the medication in the context of their treatment course [14].