Cases in Menopause

Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Data suggest that several years of systemic hormone therapy are safe in mutation carriers who have intact breasts and no personal history of...
Steven R. Goldstein, MD, CCD, NCMP
Dr. Goldstein is Professor of Obstetrics and Gynecology at New York University School of Medicine and Director of Gynecologic Ultrasound and Co-Director of Bone Densitometry and Body Composition at New York University Medical Center in New York City. He serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he serves on the gynecology advisory boards of Amgen and Pfizer.

These latest developments in bone health add to our knowledge of assessing fracture risk and treating low bone mass
In this Article
More than 9 million American women are estimated to have osteoporosis, making it the most common bone disease and an especially prevalent health problem in postmenopausal women.1
Osteoporosis causes 2 million fractures every year, leading to major medical consequences for patients.2 These fractures are associated with significant morbidity and mortality, often requiring the extended use of long-term care facilities and causing severe disability.
With a rapidly increasing elderly population, the cost of care for osteoporosis is estimated to rise to $25.3 billion by 2025.3 The medical and financial impacts of osteoporosis underscore the need for timely screening and diagnosis and the implementation of effective prevention and treatment strategies. As women’s health care providers, we are the first line of screening and diagnosis and implementation of effective treatment strategies.
In this “Update on Osteoporosis,” I discuss:
Can zoledronic acid or denosumab counter bone loss associated with aromatase inhibitors?
Majithia N, Atherton PJ, Lafky JM, et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy: a 5-year follow-up [published online ahead of print August 23, 2015]. Support Care Cancer. doi:10.1007/s00520-015-2915-2.
Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443.
Every gynecologist and women’s health care provider knows that breast cancer is a prevalent disease. It is also likely to be the most feared entity among our patients.
Aromatase inhibitors (AIs) have been shown consistently to provide benefit for patients with hormone-positive breast cancer and frequently are incorporated into treatment in both the adjuvant and metastatic settings. By inactivating the enzyme responsible for converting androgens to estrogens, AIs reduce plasma estrogen levels. This effect is helpful in the treatment of breast cancer, but it also has consequences for bone mineral density (BMD).
Estrogen promotes the inactivation of osteoclasts, thereby minimizing bone mineral resorption. When plasma levels of estrogen are suppressed, women are susceptible to loss of BMD and development of osteoporosis. This adverse effect has been observed in several clinical trials.4,5
Study focused on women with low bone mass
Majithia and colleagues set out to explore whether zoledronic acid would prevent loss of BMD in postmenopausal women with preexisting osteopenia or osteoporosis who were initiating adjuvant therapy with the AI letrozole for primary breast cancer.
Sixty postmenopausal women with estrogen-receptor–positive breast cancer and a BMD T-score of –2.0 or less were enrolled. Participants received letrozole 2.5 mg and vitamin D 400 IU daily, calcium 500 mg twice daily, and IV zoledronic acid 4 mg every 6 months for a maximum of 5 years or until disease progression. BMD at the lumbar spine and femoral neck was recorded at the start of the study and annually for 5 years. Patients were evaluated for fractures every 6 months for the duration of the trial.
Findings of Majithia and colleagues. After 5 years of therapy, mean BMD increased by 11.6% (P = .01) at the lumbar spine and by 8.8% (P = .01) at combined sites. Femoral neck BMD increased by 4.2%, although this increase was not significant (P = .23). At the end of the trial, BMDs were consistent with osteoporosis in 7% and osteopenia in 36% of patients. A total of 6 fractures were reported after 417 individual assessments.
Investigators concluded that zoledronic acid appears to prevent further bone loss in postmenopausal breast cancer patients with osteopenia or osteoporosis starting treatment with letrozole. These findings support concurrent initiation of bisphosphonate and AI therapy in this high-risk population.
Denosumab significantly delayed time to first clinical fracture
Gnant and colleagues performed a prospective, double-blind, placebo-controlled, phase 3 trial in which postmenopausal patients with early hormone-receptor– positive breast cancer undergoing treatment with an AI were randomly assigned, in a 1:1 ratio, to denosumab 60 mg or placebo administered subcutaneously every 6 months. The endpoint was time from randomization to first clinical fracture. A total of 3,420 patients were enrolled and studied over 7 years.
Findings of Gnant and colleagues. Patients given denosumab had a significantly delayed time to their first clinical fracture (hazard ratio [HR], 0.50; 95% confidence interval [CI], 0.39–0.65), compared with those in the placebo group.
The overall lower number of fractures in the denosumab group (92 vs 176) was similar in all patient subgroups, including patients with a BMD T-score of –1 or higher at baseline (n = 1,872; HR, 0.44; 95% CI, 0.31–0.64; P<.0001) and those with a BMD T-score greater than –1 at baseline (n = 1,548; HR, 0.57; 95% CI, 0.40–0.82; P = .002).
The incidence of adverse events in the safety analysis set (all patients who received at least one dose of the study drug) did not differ between the denosumab (1,366 events, or 80%) and placebo groups (1,344 events, or 79%); nor did the numbers of serious adverse events (521 vs 511, or 30% in each group). The main adverse events were arthralgia and other AI-related symptoms; no additional toxicity from the study drug was reported. Despite proactive adjudication of every potential case of osteonecrosis of the jaw by an international expert panel, no cases were reported.
Differences between the 2 studies
The study with zoledronic acid looked at BMD in a small number of patients with low bone mass over a 1-year time frame. The denosumab study was extremely large and looked at clinical fractures in women with normal as well as low bone mass.
How long should bisphosphonate therapy be continued?
Adler RA, Fuleihan GE, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment. Report of a task force of the American Society for Bone and Mineral Research [published online ahead of print September 9, 2015]. J Bone Miner Res. doi:10.1002/jbmr.2708.
An osteoporotic fracture occurs every 3 seconds worldwide, and 1 in 3 women will experience a fragility fracture after age 50.6,7 Solid evidence from randomized, placebo-controlled trials of 3 to 4 years’ duration supports the efficacy of bisphosphonates in decreasing the risk of vertebral fracture (by 40%–70%), hip fracture (by 20%–50%), and nonvertebral fracture (by 15%–39%), depending on the drug, skeletal site, and individual risk profile.8 As a result, these drugs have dominated the landscape of osteoporosis therapies for the past 2 decades.
Extension trials have suggested that prolonged bisphosphonate therapy is effective in maintaining BMD as long as 10 years with alendronate, 7 years with risedronate, and 6 years with zoledronic acid, but evidence regarding fracture risk reduction with prolonged therapy is less convincing.9–11
This report from the American Society for Bone and Mineral Research (ASBMR) examines fracture reduction—not simply BMD efficacy—in 2 trials that explored long-term use of bisphosphonates.
What 2 long-term studies reveal about fracture risk
In the Fracture Intervention Trial Long-Term Extension (FLEX), postmenopausal women who received alendronate for 10 years had fewer clinical vertebral fractures than those who switched to placebo after 5 years.
In the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) study extension, women who received 6 annual infusions of zoledronic acid had fewer morphometric vertebral fractures than those who switched to placebo after 3 years.
A hip T-score between –2 and –2.5 in FLEX and below –2.5 in the HORIZON extension predicted a beneficial response to continued therapy. Therefore, the ASBMR task force suggests that after 5 years of oral bisphosphonate or 3 years of intravenous therapy, risk reassessment should be considered.
In women at high risk for fracture (such as those who are older, have a low hip T-score or high fracture risk score, have a history of major osteoporotic fracture, or have experienced a fracture during therapy), continuation of treatment for as long as 10 years (oral) or 6 years (intravenous), with periodic evaluation, should be considered.
The ASBMR task force also found that the risk of atypical femoral fracture—but not osteonecrosis of the jaw—clearly increases with the duration of bisphosphonate therapy. However, such rare events are outweighed by vertebral fracture risk reduction in high-risk patients. For women who do not have a high fracture risk after 3 to 5 years of bisphosphonate therapy, a drug holiday of 2 to 3 years can be considered.
The ASBMR task force acknowledged that its suggested approach for long-term bisphosphonate use is based on limited evidence and was studied only for vertebral fracture reduction in a population that was mostly white and postmenopausal. This approach does not replace the need for clinical judgment. The task force also points out that future trials are unlikely to provide data for the formulation of definitive recommendations.
What this EVIDENCE means for practice
Patients who begin oral bisphosphonate therapy should continue it for 5 years, and those who start intravenous therapy should continue it for 3 years. After that time, the decision concerning continued therapy versus a “drug holiday” requires clinical judgment that takes into account the patient’s level of risk. Notable risk factors include a continued low T-score, older age, and any previous fracture, especially if that fracture occurred during therapy.
In the pipeline: The trabecular bone score may help us refine fracture risk prediction
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions. Part 2: Trabecular bone score. J Clin Densitom. 2015;18(3):309–330.
As measured by dual-energy x-ray absorptiometry (DXA), BMD is a major determinant of bone strength and fracture risk. Although DXA BMD is considered the gold standard for the diagnosis of osteoporosis, most individuals who experience a fragility fracture will have BMD values in the osteopenic or even normal range. This observation implies that the risk of fracture depends on factors other than BMD.
A number of skeletal features other than BMD, such as bone geometry, microarchitecture, mineralization, bone remodeling, and microdamage, contribute to bone strength and overall fracture risk (FIGURE). These features and characteristics of the skeleton that influence bone’s ability to resist fracture are known as bone quality.
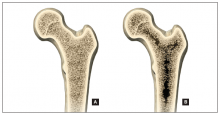
Determinants of bone strengthSkeletal features other than BMD, such as bone geometry, microarchitecture, and mineralization contribute to bone strength and overall fracture risk. This figure shows healthy microarchitecture (A) and low bone mass (B). The latter is characterized by fewer and thinner trabeculae and thin cortical bone.
Important aspects of bone quality—namely, bone microarchitecture and bone remodeling—can be assessed in bone biopsies by histomorphometry and microcomputed tomography. However, iliac crest bone biopsy is an invasive, not widely available procedure, now used primarily as a research tool.
Alternatively, a number of noninvasive imaging modalities, including quantitative computed tomography (QCT) and high-resolution magnetic resonance imaging, can measure bone geometry, microarchitecture, and bone strength and distinguish between individuals with and without fragility fracture. However, compared with standard DXA, these technologies have higher cost, a greater dose of ionizing radiation (QCT), and limited accessibility.
A major challenge, therefore, has been to incorporate into clinical practice a readily available, noninvasive technology that permits improvement in fracture risk prediction beyond that provided by the combination of standard DXA measurements and clinical risk factors. To this end, the trabecular bone score (TBS), a gray-level textural index derived from the lumbar spine DXA image, has been investigated.
How TBS assessment works
The report by Silva and colleagues comes from a task force of the International Society for Clinical Densitometry. TBS is a textual index that evaluates pixel gray-level variations in the lumbar spine DXA image, providing an indirect index of trabecular architecture.
A dense trabecular structure produces a 2-dimensional image with a large number of pixel-value variations of small amplitude and, consequently, a high TBS value. Conversely, a 2-dimensional projection of deteriorated bone architecture produces an image with a low number of pixel-value variations of high amplitude and, therefore, a low TBS.
TBS is measured in the same region of interest as the lumbar spine BMD measurement by dedicated software (TBS iNsight; Medimaps, Plan-les-Ouates, Switzerland). TBS can be obtained from lumbar spine DXA images acquired using the latest generations of GE Lunar (Madison, Wisconsin) or Hologic (Bedford, Massachusetts) densitometers, such as Prodigy and iDXA or Delphi, Horizon, QDR 4500, and Discovery.
The TBS result (which is unitless) is given for each vertebra and for the total lumbar spine (L1–L4). Abnormal vertebrae, including fractured vertebrae and vertebrae with osteoarthritic changes, can be excluded from the TBS analysis, as is done for the BMD measurement.
Silva and colleagues conclude that the ability of TBS to predict fracture risk is partially independent of central DXA BMD, clinical risk factors, and fracture probability estimated by FRAX. Based on these findings, TBS may be used to assess fracture risk in clinical practice and can be used in association with FRAX and BMD to adjust FRAX probability of fracture, guiding treatment decisions.
TBS should not be used alone to determine treatment recommendations, and it is not useful for monitoring bisphosphonate treatment in postmenopausal women with osteoporosis.
Is sarcopenia an important piece of the bone health equation?
He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis [published online ahead of print August 5, 2015]. Osteoporosis Int.
Sarcopenia is the age-associated loss of muscle mass and strength, and it has a multifactorial basis, including sedentary lifestyle, changing endocrine function, chronic disease, inflammation, insulin resistance, and nutritional deficiency. Sarcopenia may result in adverse outcomes such as physical disability, poor quality of life, escalated costs of health care, and increased mortality. The prevalence of sarcopenia is reported to range from 5% to 13% in adults aged 60 to 70 years and from 11% to 50% in people older than 80 years.12
The pathophysiology and etiology of sarcopenia and osteoporosis, and the relationship between them, are complicated and multifactorial. Recent studies have shown that muscle and bone share some common genetic, nutritional, lifestyle, and hormonal determinants, and that body composition and muscle strength are correlated with bone density.13,14 In the elderly, decreased muscle mass and increased fat mass may contribute to difficulties with physical function.
Exploring the relationship between sarcopenia and osteoporosis
He and colleagues investigated this relationship in a cohort of 17,891 people. Lean mass and grip strength were positively associated with BMD. People with sarcopenia were twice as likely as individuals without sarcopenia to have osteoporosis.
People of black, white, and Chinese heritage were analyzed. Sarcopenia was defined by relative appendicular skeletal muscle mass (RASM) cut points. RASM is calculated as lean mass (as measured by DXA) divided by height squared. For this study, He and colleagues defined sarcopenia as RASM more than 2 standard deviations below the mean of young male and female reference groups. The current objective cut points for sarcopenia in men and women are RASM of 7.26 kg/m2 or less and RASM of 5.45 kg/m2 or lower, respectively.
These criteria for sarcopenia are based on previous studies in people of white and black race.15,16 Because of ethnic differences in body composition, these criteria do not appear to be applicable to Chinese individuals. An earlier study17 established the cutoff values of 6.08 kg/m2 and 4.79 kg/m2 for sarcopenia in healthy Chinese men and women, respectively, and these criteria were used for the diagnosis of sarcopenia in the Chinese sample.
Fat mass also was measured by DXA. BMD was positively associated with lean mass and negatively associated with fat mass. Grip strength was significantly associated with a higher BMD. Each standard deviation increase in RASM resulted in a risk reduction of approximately 37% for osteopenia or osteoporosis (odds ratio [OR], 0.63; 95% CI, 0.59−0.66).
Individuals with sarcopenia, as defined by RASM, were twice as likely as patients without sarcopenia to have osteopenia or osteoporosis (OR, 2.04; 95% CI, 1.61−2.60). Similarly, people with sarcopenia (low muscle mass and grip strength) were approximately 1.8 times more likely than individuals with normal muscle mass and grip strength to have osteopenia or osteoporosis (OR, 1.87; 95% CI, 1.09−3.20).
He and colleagues concluded that high lean mass and muscle strength were positively associated with BMD. Sarcopenia is associated with low BMD and osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.

Data suggest that several years of systemic hormone therapy are safe in mutation carriers who have intact breasts and no personal history of...

The latest guidance on safe use of menopausal hormone therapy
A new agent, and new understandings of old drugs, top the year’s developments in bone health
Easy to use and effective, this recently approved agent appears well suited to ObGyn office practice
