CHALLENGE 4: Nonfundal IUD location
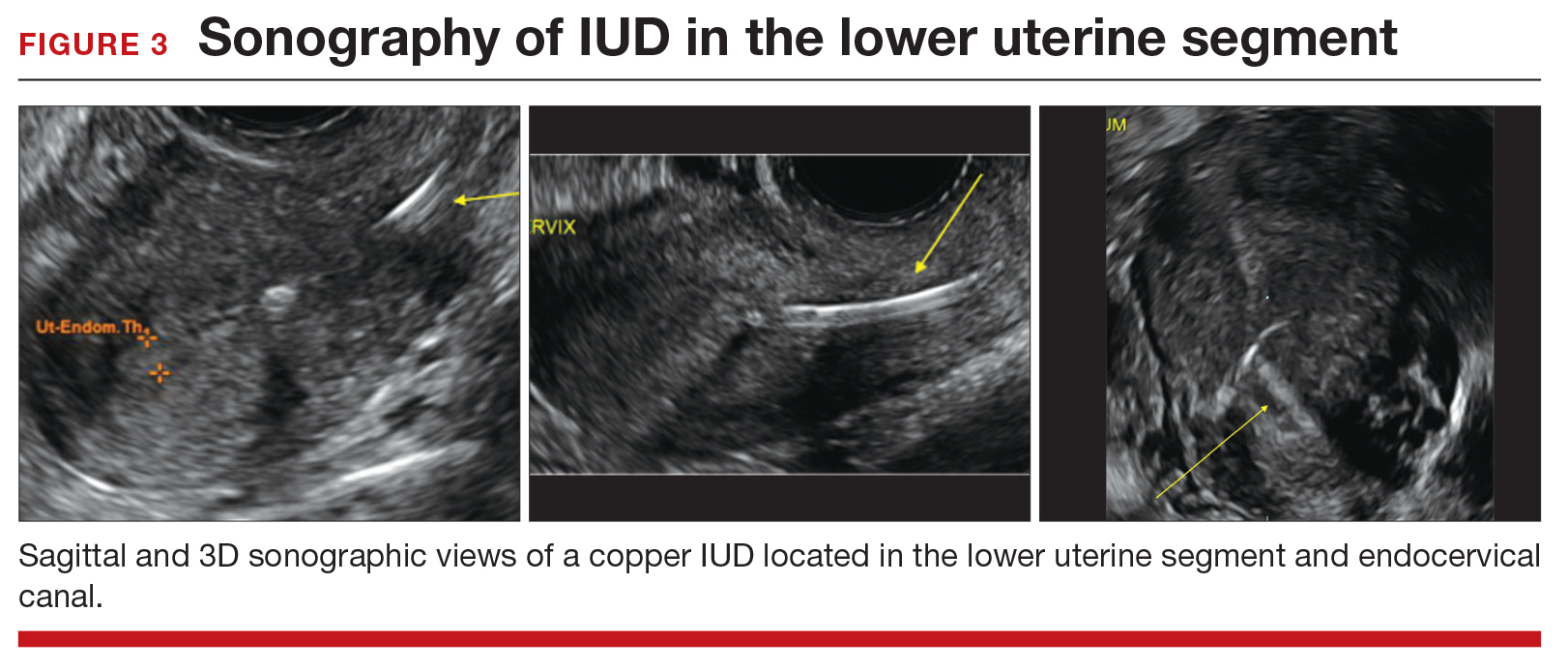
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.
Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.