Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.
Steps in the procedure
Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).
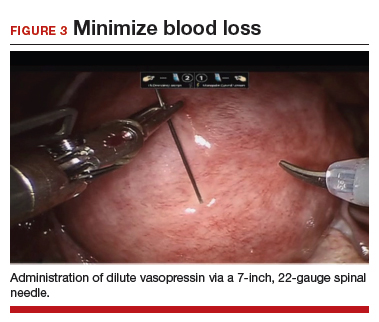
To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28
Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...