Case 1 Induction at 39 weeks in a healthy nulliparous woman
A healthy 35-year-old woman (G1P0) at 39 weeks 0 days and with an uncomplicated pregnancy presents to your office for a routine prenatal visit. She inquires about scheduling an induction of labor, noting that she read a news story about induction at 39 weeks and that it might lower her chance of having a cesarean delivery (CD).
You perform a cervical exam—she is 1 cm dilated, 3 cm long, -2 station, posterior, and firm. You sweep her membranes after obtaining verbal consent. After describing the induction process, you explain that she might be hospitalized for several days before the birth given the need for cervical ripening. “You mean I need to stay in the hospital for the entire process?” she asks incredulously.
Over the past 20 years, the percentage of patients undergoing induction of labor (IOL) has increased from 10% to 25%.1 This percentage likely will rise over time, particularly in the wake of a recent randomized controlled trial suggesting potential maternal benefits, such as reduced CD rate, for nulliparas induced at 39 weeks compared with expectant management.2 Although there have not been any changes to guidelines for timing of IOL from such professional societies such as the American College of Obstetricians and Gynecologists (ACOG) or the Society for Maternal-Fetal Medicine, key considerations of rising IOL volume include patient experience, labor and delivery (L&D) units’ capacity and resources, and associated health care costs.
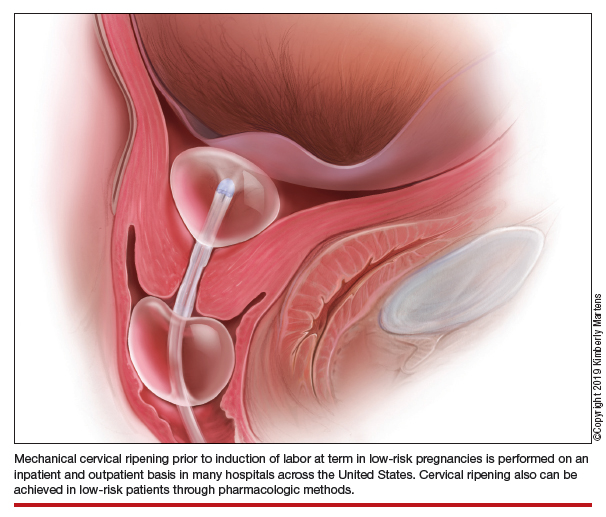
An essential part of successful induction involves patience. Induction can be a lengthy process, particularly for nulliparas with unripe cervices. Cervical ripening is a necessary component of successful labor induction, whether achieved mechanically or pharmacologically with synthetic prostaglandins, and it has been shown to lower the chance of CD.3,4 However, achieving a ripe cervix is often the lengthiest part of an induction, and not uncommonly consumes 12 to 24 hours or more of inpatient time. Investigators have sought ways to make this process more expeditious. For example, the FOR-MOMI trial demonstrated that the induction-to-delivery time was several hours shorter when cervical ripening combined mechanical and pharmacologic approaches (Foley balloon plus misoprostol), compared with either method alone, without any increase in maternal or fetal complication rates.5
Better yet, what if admission to the L&D unit for IOL at term could be deferred until the cervix is ripe? A number of hospitals in the United States have successfully introduced outpatient cervical ripening, and several small observational and randomized controlled trials have reported good results in terms of safety, efficacy and time saved, and patient experience. Here, we will make the case that outpatient cervical ripening should be the standard of care for low-risk pregnancies.
Mechanical cervical ripening
Safety
Although data are limited on the safety, the authors of an ACOG Practice Bulletin suggest that, based on the available evidence of mechanical ripening in an inpatient setting, it is also appropriate in the outpatient setting.6 Unlike cervical ripening using prostaglandins, mechanical ripening is not associated with tachysystole, fetal intolerance of labor, or meconium staining.3 A cohort study of nearly 2,000 low-risk patients who underwent Foley catheter placement for cervical ripening using an outpatient protocol but monitored overnight as inpatients and evaluated for adverse outcomes found no CD for fetal distress, vaginal bleeding, placental abruption, or intrapartum stillbirth.7 The authors posited that, given this safety profile in the inpatient setting, that mechanical cervical ripening with a Foley catheter would be appropriate for outpatient use in low-risk populations. Other systematic reviews have been reassuring as well, with exceedingly low complication rates during inpatient mechanical cervical ripening.8 These data advocate for the evaluation of cervical ripening in the outpatient setting.
The evidence for outpatient mechanical ripening, although again limited, also has demonstrated safety. There does not appear to be an increased rate of maternal or neonatal complications, including infectious morbidity, postpartum hemorrhage, CD, operative vaginal delivery, or fetal distress.9-12
Continue to: Efficacy and length-of-stay...