Our approach to mechanical cervical ripening
Most patients undergoing scheduled IOL are reasonable candidates for outpatient cervical ripening based on safety and efficacy. By definition, scheduling in advance implies that the provider has determined that outpatient management is reasonable until that date, and the plan for outpatient ripening need not prolong this period.
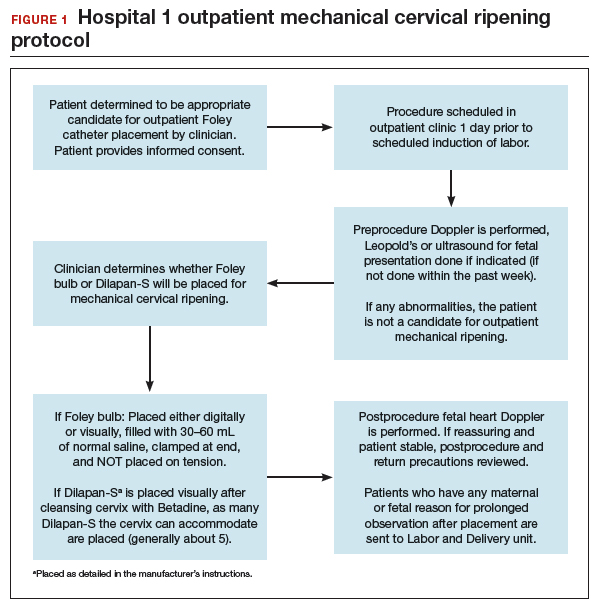
FIGURES 1 and 2 show protocols for our 2 hospital centers, which regularly allow for outpatient mechanical cervical ripening. In the process of protocol development, we identified absolute and relative contraindications to determine appropriate candidates. We exclude women who require inpatient management of medical or obstetric conditions (for example, women with severe preeclampsia or any condition requiring continuous fetal monitoring). We also do not routinely recommend outpatient cervical ripening to patients who do not have the necessary social conditions to make this process as safe as possible (including stable housing, reliable transportation, and a support person), although this occurs with some exceptions depending on individual patient situations.
Some examples of ideal candidates for outpatient mechanical cervical ripening include those undergoing elective or routine prolonged gestation inductions, or inductions for well-controlled, stable conditions (chronic hypertension and gestational diabetes). At one center, after thorough counseling and assessment, outpatient cervical ripening is also offered to patients with mild risk factors, including twins, prior low transverse CD, stable preeclampsia without severe features, isolated oligohydramnios with otherwise reassuring fetal status, and other similar conditions.
After mechanical cervical ripening placement (either Foley catheter or mechanical dilators), the clinician completes a postprocedure safety checklist and detailed procedure documentation, including number and type of foreign bodies placed. If there are any concerns regarding maternal or fetal well-being, the patient is sent to L&D for evaluation. If the procedure was tolerated well, the patient is discharged home, after a reactive postprocedure nonstress test is done, with detailed instructions for self-care, as well as with a list of symptoms that warrant prompt evaluation prior to scheduled induction time. In a large California hospital group following a similar protocol, only about 5% of women presented in labor before their scheduled induction.18
Case 2 Cervical ripening for labor preparation in low-risk pregnancy
A 32-year-old woman (G1P0) with an uncomplicated pregnancy at 40 weeks and 3 days presents to your office for a routine prenatal visit. Her vital signs are normal, and her fetus is vertex with an estimated fetal weight of 7.5 lb by Leopald’s maneuvers. You perform a cervical exam and find that her cervix is closed, long, and posterior.
You discuss with her your recommendation for induction of labor by 41 weeks, and she agrees. You also discuss the need for cervical ripening and recommend misoprostol given her closed cervix. You explain that several doses may be needed to get her cervix ready for labor, and she asks, “Do I have to stay in the hospital that whole time?”
Pharmacologic cervical ripening
Efficacy
There are multiple pharmacologic agents that can be used for ripening an unfavorable cervix. The main agents used in the United States are prostaglandins, either PGE1 (oral or vaginal misoprostol) or PGE2 in a gel or sustained-release vaginal insert (dinoprostone).
Outpatient misoprostol to avoid labor induction. Many studies have looked at outpatient misoprostol use as a “prophylactic measure” (to prevent the need for labor induction). For example, Gaffaney and colleagues showed that administering outpatient oral misoprostol (100 µg every 24 hours for up to 3 doses) after 40 weeks’ gestation to women with an unfavorable cervix significantly decreased the time to delivery by a day and a half.19 Similarly, PonMalar and colleagues demonstrated that administering 25 µg of vaginal misoprostol in a single dose as an outpatient after stripping the membranes significantly reduced time to delivery by 2 days.20 And Stitely and colleagues found a significant reduction in the need for labor induction with the use of outpatient vaginal misoprostol. They administered up to 2 doses of misoprostol 25 µg vaginally every 24 hours for the 48 hours prior to a scheduled postdates induction and found a large reduction in the need for labor induction (11% vs 85%; P<.01).21
Continue to: Multiple protocols and regimens...