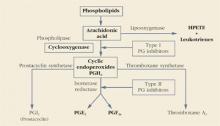
Prostaglandins are made throughout the body and are important autocrine and paracrine regulators of cellular and organ function. As depicted below, the main substrate for their production is arachidonic acid, a major constituent of cell walls. Under some circumstances, phospholipase A2 also can be used as a substrate for prostaglandin production.
Cyclooxygenase, also called prostaglandin H synthase, is the first enzymatic step in the conversion of arachidonic acid into prostaglandins. This enzyme folds the arachidonic acid molecule (cyclization) and oxygenates it to produce prostaglandin H2 (PGH2).
All other members of the prostaglandin family are then formed from PGH2. Arachidonic acid also is the substrate for the production of leukotrienes and 5-hydroxyeicosatetraenoic acid (5-HETE) through the 5-lipooxygenase pathway. Like prostaglandins F2α and E2, the products of the lipooxygenase pathway are potent vasoconstrictors and stimulators of uterine contractions.—Roger P. Smith, MD, and Jeffrey Ellis, MD
Increased uterine activity was first hypothesized as a cause of dysmenorrhea in 1932. By the late 1930s, objective findings began to support that hypothesis.1 The correlation between uterine activity and menstrual pain was strengthened when Jacobson et al studied simultaneous electrical and mechanical changes within the uterus.2,3 Using an intrauterine air-filled balloon system, Wilson and Kurzrok also noted the relationship between maximal uterine activity and pain.4,5 Despite the strength of these investigations, few changes occurred in the way dysmenorrhea was viewed or treated.
In the late 1940s, Liessé demonstrated that women with dysmenorrhea not only had a greater degree of uterine electrical and mechanical activity, but that this activity correlated with the pain of menstruation.6 Liessé found minimal activity between pains and as many as 30 irregular electrical discharges per second during pain, suggesting a cause (electrical) for dysmenorrhea but offering no clue to the underlying physiologic disturbance that might account for it.
The most detailed and influential studies of dysmenorrhea and uterine activity came in 1947 in a trial conducted by Woodbury.7 His findings of a direct correlation between pressure, pattern of contractions, resting tone, and pain became a standard reference.
After Woodbury, the stage was set for a connection to be made between uterine activity and prostaglandins. That happened in 1965, when Pickles reported elevated levels of prostaglandin F2αin the menstrual fluid of dysmenorrheic women.8
During the past 2 decades, more sophisticated analytic techniques have been applied to intrauterine pressure data,9,10 and strong correlations between uterine activity and pain have been reported.11,12 The basic assertion that menstrual pain is caused by increased intrauterine pressure, poor relaxation, and more frequent, irregular contractions appears to be valid.—Roger P. Smith, MD, and Jeffrey Ellis, MD
REFERENCES
1. Novac E, Reynolds SRM. The cause of primary dysmenorrhea. JAMA. 1932;99:1466.-
2. Jacobson E, Lackner JE, Sinykin MB. Electrical and mechanical activity of the human non-pregnant uterus. Am J Obstet Gynecol. 1939;38:1008.-
3. Jacobson E, Lackner JE, Sinykin MB. Activity of the human non-pregnant uterus. Am J Physiol. 1940;53:407.-
4. Wilson L, Kurzrok R. Studies on the motility of the human uterus in vivo. Endocrinology. 1938;23:79.-
5. Wilson L, Kurzrok R. Uterine contractility in functional dysmenorrhea. Endocrinology. 1940;27:23.-
6. Liessé A. L’Activité électrique de l’uterus dans la dysmenorrhee functionnelle. Gynec et Obstet. 1948;47:850.-
7. Woodbury RA, Torpin R, Child GP, Watson H, Jarboe M. Myometrial physiology and its relation to pelvic pain. JAMA. 1947;134:1081-1085.
8. Pickles VR, Hall WJ, Best FA, Smith GN. Prostaglandins in endometrium and menstrual fluid from normal and dysmenorrheic subjects. J Obstet Gynaecol Br Comm. 1965;72:185.-
9. Smith RP. Intrauterine pressure analysis in nonpregnant dysmenorrheic women. Med Instr. 1984;185:137-139.
10. Smith RP. Distribution analysis of intrauterine pressure in nonpregnant dysmenorrheic women. Am J Obstet Gynecol. 1984;150:271-273.
11. Smith RP. The dynamics of nonsteroidal anti-inflammatory therapy for primary dysmenorrhea. Obstet Gynecol. 1987;70:785-788.
12. Smith RP, Powell JR. Simultaneous objective and subjective evaluation of meclofenamate sodium in the treatment of primary dysmenorrhea. Am J Obstet Gynecol. 1987;157:611-616.
Classes of NSAIDs
Although aspirin was synthesized in 1853 and incorporated into medical practice in 1899, its history goes back even farther. That fact—along with the introduction of newer agents—ensured that NSAIDs became the mainstays of medical therapy for fever, pain, and inflammation. In the United States, NSAIDs are among the most widely prescribed drugs, with more than 70 million prescriptions and more than 30 billion over-the-counter tablets sold each year.20 Most of their therapeutic effects come from their ability to inhibit the production of prostaglandins.21
Interestingly, some drugs with the ability to inhibit prostaglandin synthesis have little clinical usefulness. Some have weak antiprostaglandin activity, require metabolic transformation to become active, or have side effects that limit their usefulness. While these drugs can be used to treat dysmenorrhea, they generally have been replaced by more effective agents.