Details of the trial
Eligible patients were previously enrolled in a randomized, clinical trial investigating the efficacy of InterStim. Of the 23 sites that participated in the original study, only 17 elected to participate in the follow-up trial.
One hundred fifty-two subjects were enrolled—129 of them crossing over from the original study and 23 newly recruited and implanted with the InterStim device ( FIGURE 3 ). Investigators reviewed data from follow-up visits, self-reported symptoms obtained from voiding diaries collected annually for 5 years, and descriptive summaries of adverse events related to the sacral nerve stimulator.
One-year data were available on 138 subjects, and 5-year data were available on 105. Of the 47 participants who did not participate in 5-year follow-up, 16 had the InterStim device removed.
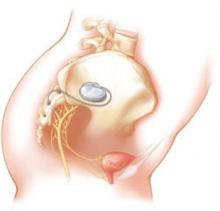
FIGURE 3 Aim of InterStim therapy is to regulate brain–bladder signals
The InterStim device sends a mild electrical impulse through a lead to the sacral nerves to influence the bladder and surrounding muscles. Sacral nerve stimulation helps regulate interaction between the brain and bladder and reduce voiding dysfunction.
Additional surgery was performed in half of patients
who had an adverse event
No life-threatening or irreversible adverse events were reported during the 5 years of follow-up. However, 221 adverse events occurred, of which 110 required a minor surgical procedure—in some cases, more than one. The most commonly reported adverse event was new pain or pain at the implantation site (46.1%). The most common surgical procedure was device exchange (23.7%).
Because many patients who suffer from overactive bladder syndrome fail to respond to conservative therapies, such as behavior modification and pharmacotherapy, novel alternatives are needed. This study:
- contributes to our understanding of sacral nerve stimulation
- provides much needed data on the long-term safety and efficacy of InterStim therapy
- proposes that InterStim therapy is safe and efficacious for women who have refractory overactive bladder syndrome
- suggests that it may become the gold standard for treatment of this condition.
At the Cleveland Clinic, we offer sacral nerve stimulation for refractory overactive bladder and nonobstructive urinary retention.