Commentary
I perform extracorporeal morcellation; Better vaginal surgery training needed; A patient's perspective
Letters from readers, and a patient, on morcellation
Q&A with Ray A. Wertheim, MD, and Harry Reich, MD
Dr. Wertheim and Dr. Reich report no financial relationships relevant to this article.

A recent FDA hearing on the use of this technology has cast a cloud over its future. Here, two members of the AAGL Tissue Extraction Task Force discuss the evidence and emphasize the importance of preserving minimally invasive options in women’s health.
Visit the Morcellation Topic Collection Page for additional articles, videos, and audiocasts.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In February 2014, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Not surprisingly, the numerous statements and warnings since then have led to some confusion in the specialty about the safest course of action for tissue extraction during hysterectomy and myomectomy in women with a large uterus.
To explore the options more deeply and address the future of minimally invasive surgery (MIS) in women’s health, OBG Management invited two experts to comment: Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
Both surgeons believe that power morcellation should remain an option for selected cases, though neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be utilized whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?2 How does it compare with the ACOG and FDA statements on the use of power morcellation?
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. I’ve been practicing for almost 40 years in academic and private settings, and I found this group to be the brightest, most caring and compassionate group with whom I’ve ever worked. Our review is the most complete report to date, more comprehensive than the reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL task force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. One of the problems we encountered was that there are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction in individual patients undergoing uterine surgery.
Letters from readers, and a patient, on morcellation
The FDA’s recent safety communication on the risks of laparoscopic power morcellation prompts Brigham and Women’s and Massachusetts General...
Brigham and Women’s and Massachusetts General are taking steps to eliminate most uses of open power morcellation
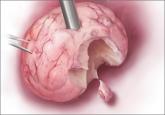
The current practice of open power morcellation is being scrutinized by those within and outside of the ObGyn community. We need to re-examine our...
The most promising alternative to open power morcellation is morcellation in a bag, described here
James D. Kondrup, MD, demonstrates his surgical team's approach to morcellation, with use of a morcellation bag after laparoscopic supracervical...
