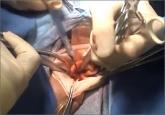
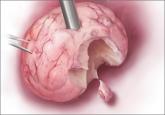
About 5.2 million people insured by Highmark Inc, a Blue Cross and Blue Shield (BCBS)–affiliated company in Pennsylvania, Delaware, and West Virginia will be denied coverage for laparoscopic power morcellation used in gynecologic surgery. Highmark is the fourth-largest BCBS affiliate.1
On August 2, spokesman Aaron Billger announced by email that, in the best interest of their members, Highmark is halting coverage as of September 1, 2014.2 This decision comes in response to the US Food and Drug Administration (FDA) Safety Communication of April 17, 2014, that discouraged the use of power morcellation during hysterectomy and myomectomy because of the increased risk of dispersion of benign myoma tissue and occult malignant tissue through the abdominal cavity.2,3
University of Pittsburgh Medical Center (UPMC), the largest hospital group in western Pennsylvania, publicized that it will stop performing laparoscopic power morcellation as of September 1. UPMC spokesperson Gloria Kreps said the decision was an “appropriate and prudent course of action,” and that the hospital was “looking to the FDA for further guidance.”4 Other medical centers and hospitals have banned the use of power morcellation, beginning with Brigham and Women’s and Massachusetts General in April.5
In April 2014, Ethicon, a unit of Johnson & Johnson, suspended sales of all its morcellation devices. In an urgent Medical Device Market Withdrawal dated July 30, 2014, Ethicon initiated a “worldwide voluntary market withdrawal of all Ethicon Morcellation Devices that currently remain on the market.”6 Ethicon will issue prorated credit for morcellation devices returned by December 30, 2014, with full credit issued for unopened, unexpired disposable products.6
The FDA Obstetrics and Gynecology Devices Panel Advisory Committee held a 2-day hearing in July 2014 to weigh the risks and benefits of power morcellation. The panel will send recommendations to the FDA, and a final decision on the use of laparoscopic power morcellation will be forthcoming.7