Expert Commentary

Why FDA hearing on morcellation safety could drive innovation
Reaction from Cheryl Iglesia, MD, Advisory Panel Member, to FDA’s 2-Day Hearing
Q&A with Ray A. Wertheim, MD, and Harry Reich, MD
Dr. Wertheim is Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia.
Dr. Reich practices gynecologic surgery in Wilkes-Barre, Pennsylvania.
Dr. Wertheim and Dr. Reich report no financial relationships relevant to this article.

A recent FDA hearing on the use of this technology has cast a cloud over its future. Here, two members of the AAGL Tissue Extraction Task Force discuss the evidence and emphasize the importance of preserving minimally invasive options in women’s health.
Visit the Morcellation Topic Collection Page for additional articles, videos, and audiocasts.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In July 2014, the FDA convened a two-day hearing of the Obstetrics and Gynecology Devices Panel (one of the panels in its Medical Devices Advisory Committee) to consider whether power morcellation should remain an option and, if so, what restrictions or labeling might be recommended.
In advance of the FDA hearing, OBG Management invited two experts in women’s health to explore the options more deeply and address the future of minimally invasive surgery (MIS): Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
Both surgeons believe that power morcellation should remain an option for selected cases, although neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be used whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?1
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. Our review is the most complete report to date, more comprehensive than the current reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL Task Force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. There are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
Related article: First large study on risk of cancer spread using power morcellation. Janelle Yates (News for your Practice; August 2014)
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction for individual patients undergoing uterine surgery.
AAGL recommendations on the use of power morcellationIn its position statement, the AAGL Tissue Extraction Task Force made the following main points, recommending that surgeons:
Further research also is needed to determine how best to diagnose sarcomas preoperatively, the task force noted. The full report is available on the AAGL Web site.1 —Ray A. Wertheim, MD |

Reaction from Cheryl Iglesia, MD, Advisory Panel Member, to FDA’s 2-Day Hearing
Letters from readers, and a patient, on morcellation
The FDA’s recent safety communication on the risks of laparoscopic power morcellation prompts Brigham and Women’s and Massachusetts General...
James D. Kondrup, MD, demonstrates his surgical team's approach to morcellation, with use of a morcellation bag after laparoscopic supracervical...
Hospitals suspend use of power morcellators until further notice
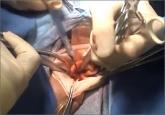
Ceana Nezhat, MD, and Erica Dun, MD, show how they perform enclosed vaginal morcellation
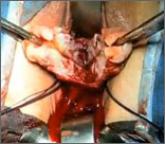
Dr. Shibley discusses a novel strategy he has developed to address the problem of tissue dispersion during open power morcellation
This technique is K. Anthony Shibley, MD's, short-term surgical solution to performing closed power morcellation. His patented pneumoperitoneum...
The most promising alternative to open power morcellation is morcellation in a bag, described here
