TREATMENT Saline and psychotropics
While in the emergency room, Mr. D receives 2 L of IV saline. His CK level falls to 137 U/L. A challenge with IV lorazepam, 2 mg, also is performed. Mr. D becomes talkative and follows commands with fluid movements, but his disorganized, delusional thoughts persist. BFCRS score has improved to 9 (Table 1). He is admitted to the psychiatric unit and started on oral lorazepam, 2 mg, 3 times daily, for catatonia, and olanzapine, 10 mg/d, for psychosis.
The differential diagnosis for Mr. D’s psychosis includes substance-induced psychotic disorder, schizophrenia, bipolar disorder, and psychosis with another organic cause (Table 2).7 Further medical workup is completed, including a urine drug screen, testing for HIV, hepatitis B, syphilis, lead and heavy metals, ceruloplasmin, vitamin B12, folate, antinuclear antibody, sedimentation rate, and brain MRI. Cannabinoids are detected in his urine drug screen. Another urine sample is sent to an outside lab to test for several synthetic drugs, including MDMA, 3,4-methylenedioxy- N-ethyl-amphetamine, 2C-B, 2C-C, 2C-I, and 2C-P, results of which also are negative.
By the second day of hospitalization, Mr. D appears less disorganized but continues to complain of “scrambled thoughts” and appears guarded. Despite initial response to IV lorazepam and its continuation in oral form, over the next day Mr. D appears more psychomotor-slowed, with motor stiffness. His score on the BFCRS increases, with significant posturing; vital signs remain stable, however.
What is your next step in managing his catatonic symptoms?
a) increase olanzapine
b) decrease olanzapine
c) decrease lorazepam
d) emergent ECT
e) switch to haloperidol
The authors’ observations
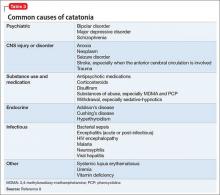
Although catatonia can be associated with a mood or psychotic disorder, it also can be induced by a medication or general medical condition (Table 3).8 It is thought that catatonia is associated with decreased γ-aminobutyric acid (GABA) and dopamine D2 receptor activity, and increased N-methyl-d-aspartate (NMDA) receptor activity.9 Antipsychotics could worsen catatonia through D2 blockade. Benzodiazepines, however, improve catatonia by increasing GABA and decreasing NMDA receptor activity. In this case, Mr. D was naïve to antipsychotics and seemed to be sensitive to them, as evidenced by his worsening symptoms.
Which condition should be considered in the differential diagnosis?
a) parkinsonian-hyperpyrexia syndrome
b) neuroleptic malignant syndrome (NMS)
c) stiff person syndrome
d) serotonin syndrome
e) CNS infection
The authors’ observations
NMS, catatonia, and parkinsonian-hyperpyrexia syndrome are all related to diminished action of dopamine at the D2 receptor. Although the mechanism of catatonia is not completely understood, NMS is thought to be caused by blockade at the D2 receptors by antipsychotics, whereas parkinsonian-hyperpyrexia syndrome is related to withdrawal of dopamine agonists. Because of the similarity in symptoms and proposed mechanisms, some experts hypothesize that NMS is a drug-induced malignant catatonia.10,11 Interestingly, NMS and catatonia respond to withdrawal of antipsychotics, and addition of benzodiazepines and ECT.
Mr. D showed posturing and other behavioral abnormalities, which are less common in NMS. Furthermore, although he had episodes of mild tachycardia, autonomic dysregulation—a hallmark of NMS—was not found. Given the common shared deficiency of activity at the D2 receptor in both NMS and catatonia, antipsychotics could cause or worsen either condition.
TREATMENT ECT
Mr. D’s olanzapine dosage is decreased to 2.5 mg/d. His catatonic symptoms improve with each dosage of oral lorazepam; however, effects seem to lessen and last for shorter periods over the following day. Additionally, Mr. D again becomes more disorganized, stiff, and unable to feed or bathe himself, and develops episodes of mild tachycardia.
Given Mr. D’s partial and poorly sustained response to lorazepam, a trial of ECT is pursued. On the third day of hospitalization, he receives ECT with bi-frontal lead placement at 25% energy. Concurrently, olanzapine is discontinued because of worsening muscle stiffness and concern about neuroleptic sensitivity. His BFCRS score after ECT is 2, and he is noted to be more interactive on the inpatient unit. He continues to receive ECT 3 times a week, with notable improvement, but ongoing psychotic symptoms and catatonic symptoms partially reemerge between ECT treatments. Lead placement is changed to bi-temporal by the third treatment, and the energy setting is increased from 25% to 50%, and to 75% by the sixth treatment. An additional nighttime dose of oral lorazepam, 2 mg, is added after the sixth treatment, in an attempt to reduce “wearing off” by morning.
After the seventh treatment, Mr. D is able to maintain logical conversation without re-emergence of catatonic symptoms over 2 days, signifying a turning point in the treatment course. The ECT energy setting is decreased to 50% to minimize potential memory deficits. His insight into his illness and treatment dramatically improve over the next few days. ECT is discontinued after the tenth treatment and Mr. D is discharged home to the care of his family.