OUTCOME Gradual improvement
Mr. F is started on nicotine gum, 2 mg/d, for smoking cessation and fluoxetine, 20 mg/d, for depression, and a dietary consult is made for his poor appetite and weight loss. His psychotic symptoms continue to improve, and by hospital Day 10, his depressive symptoms begin to improve as well: his affect brightens, he has increased appetite, and he wants to shave. He also exhibits mildly increased insight into his illness.
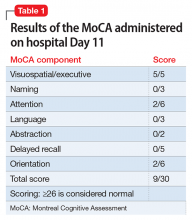
The Montreal Cognitive Assessment (MoCA) is administered on hospital Day 11 and indicates that Mr. F’s cognitive ability is severely impaired (Table 1). Over the next several days, his affect brightens, and Mr. F becomes more talkative and less withdrawn. With additional caloric intake and dietary supplements, he begins to regain weight. The MoCA is administered again on hospital Day 16 and shows significant neurocognitive improvement in organization of thought (as evidenced by the increased scores in naming, language, and orientation); however, moderate impairment is still present (Table 2).By hospital Day 20, Mr. F’s hallucinations are resolved, his delusions are greatly attenuated, and his mood is improved. Although his cognition is still mildly impaired, it is greatly improved compared with when he was first admitted. A third MoCA administered on the day of discharge (hospital Day 24) is scored at 21/30 (≥26 is considered normal). Mr. F has become more socially interactive, participating in group therapy sessions on multiple occasions. He also has improved insight into his illness, understanding the need for medication and close follow-up.Mr. F is discharged with risperidone, 2 mg twice daily, for schizophrenia, fluoxetine, 20 mg/d, for depression, and trazodone, 50 mg, as needed, for sleep, and is referred to a community mental health center for comprehensive follow-up, including vocational rehabilitation and social skills training.
The authors’ observations
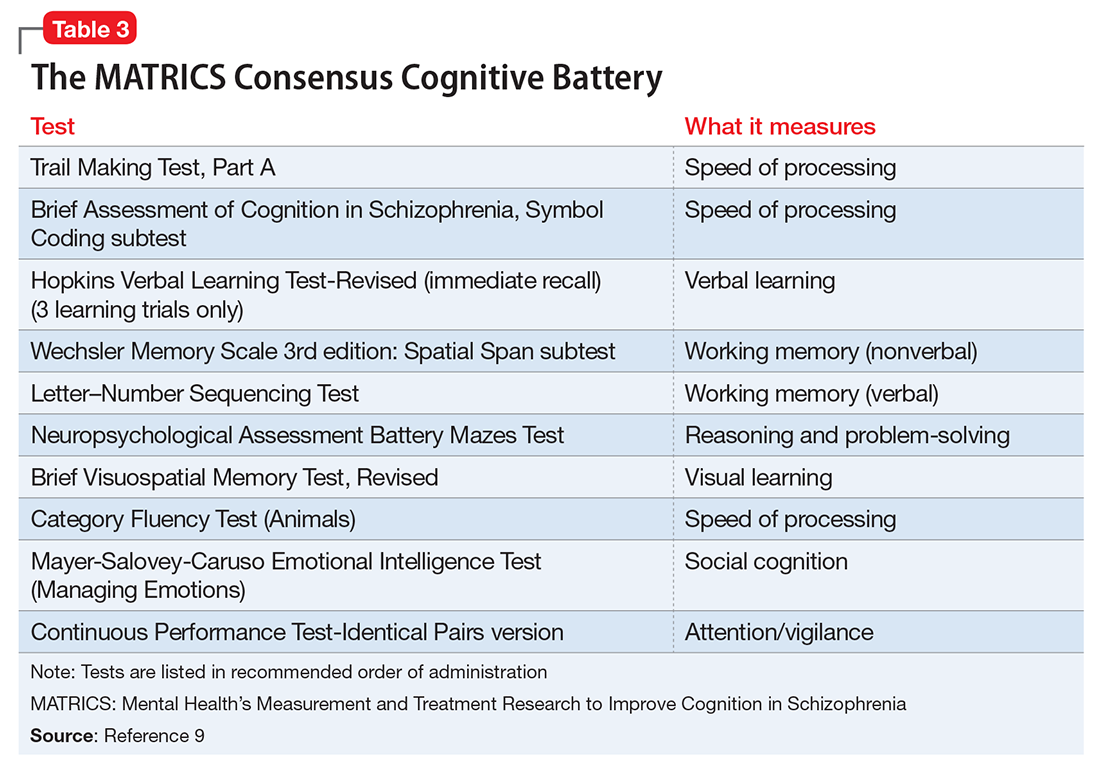
A major goal of the National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was to develop a consensus cognitive battery for clinical trials of cognition-enhancing treatments for schizophrenia. The MATRICS Consensus Cognitive Battery (MCCB) is a comprehensive cognitive assessment designed for use in patients with schizophrenia (Table 39). Although the MCCB was developed to be the standard tool for assessing cognitive change in clinical trials of cognition-enhancing drugs for schizophrenia, it also may aid evaluation of cognitive remediation strategies.9
In Mr. F’s case, such testing was not performed, in part because of his improvement. The MoCA was chosen because it is a universally accepted brief cognitive assessment tool used for screening. More robust testing can be administered by the neuropsychiatry team if indicated and if resources are available.