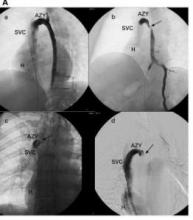
The notion that MS might stem from a narrowing or blockage of one or more veins that direct blood out of the brain — a condition known as chronic cerebrospinal venous insufficiency (CCSVI) — led to immense interest by MS patients in endovascular treatments aimed at opening these veins to improve blood outflow and hopefully relieve their symptoms. The condition was identified and named by Dr. Paolo Zamboni, a vascular surgeon at the University of Ferrara, Italy.
The CCSVI Alliance, among other groups, provides patients with a central source for information.
Endovascular treatments aimed at relieving CCSVI and possibly resolving MS symptoms have also attracted some physician enthusiasts. Speaking during a session devoted to this topic at the International Symposium on Endovascular Therapy (ISET) in Miami Beach last week, Dr. Salvatore J.A. Sclafani briefly described his own recent story. Last spring, he resigned as chairman of radiology at Downstate Medical Center in Brooklyn because he was thwarted by his hospital’s investigational review board from conducting the CCSVI treatment study he designed. He left for a nearby ambulatory-care center where, he said, he can now treat and study 1,000 patients with CCSVI a year and gather the information he feels is needed before a randomized, controlled trial should start.
But others who spoke during the ISET session said it was a mistake for interventionalists to continue to open veins in MS patients outside of formal, controlled trials.
“It’s a slippery slope to treat patients based on their anatomy alone,” said Dr. Michael Dake, a professor of cardiothoracic surgery at Stanford and the first U.S. physician to treat CCSVI in Spring 2009. “I want to know if a patient with MS has CCSVI and if the narrowing is successfully treated is it possible to objectively demonstrate physiologic improvement in relevant parameters and an associated relief of symptoms. New metrics are needed to address the effects of treatments on new targets. We see lots of patients with venous problems who don’t have MS. It’s time to look [for evidence} in a randomized trial.”
The unfortunate truth is that in this setting, as often happens in medicine, many affected patients don’t share the prudence and patience of researchers like Dr. Dake.
“Many patients do not want to wait for a lengthy trial. They’ll seek treatment on their own,” said Sharon Richardson, president of the CCSVI Alliance and a MS patient who says she received some symptom relief after receiving a pair of vein-opening stents in a procedure done by Dr. Dake.
—Mitchel Zoler (on Twitter @mitchelzoler)