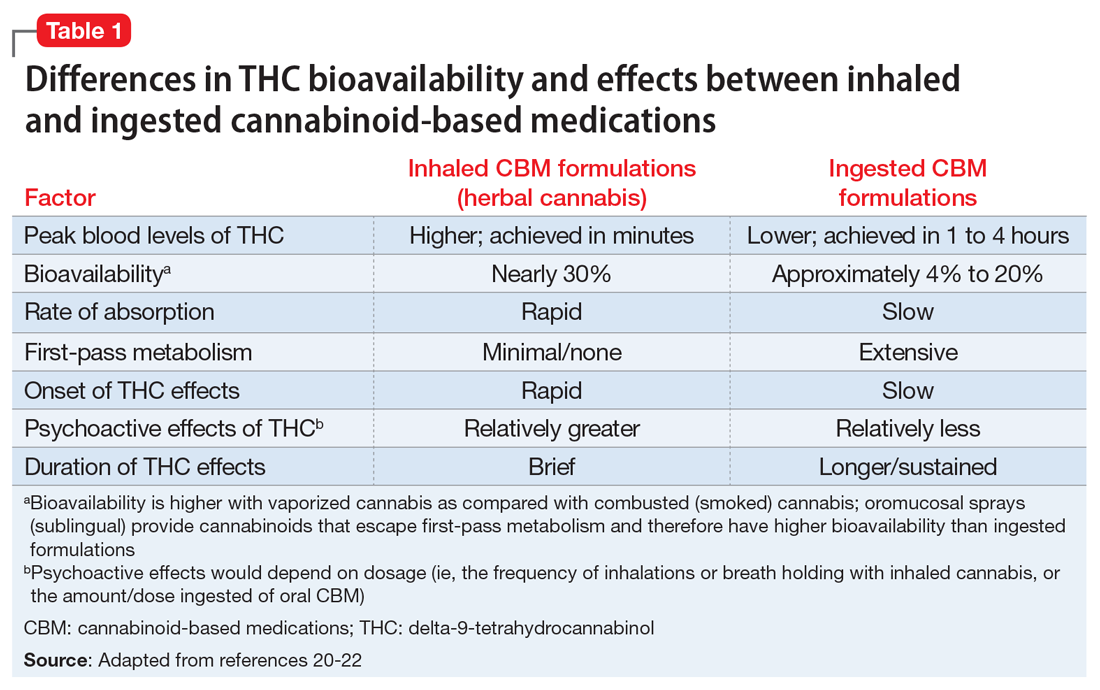
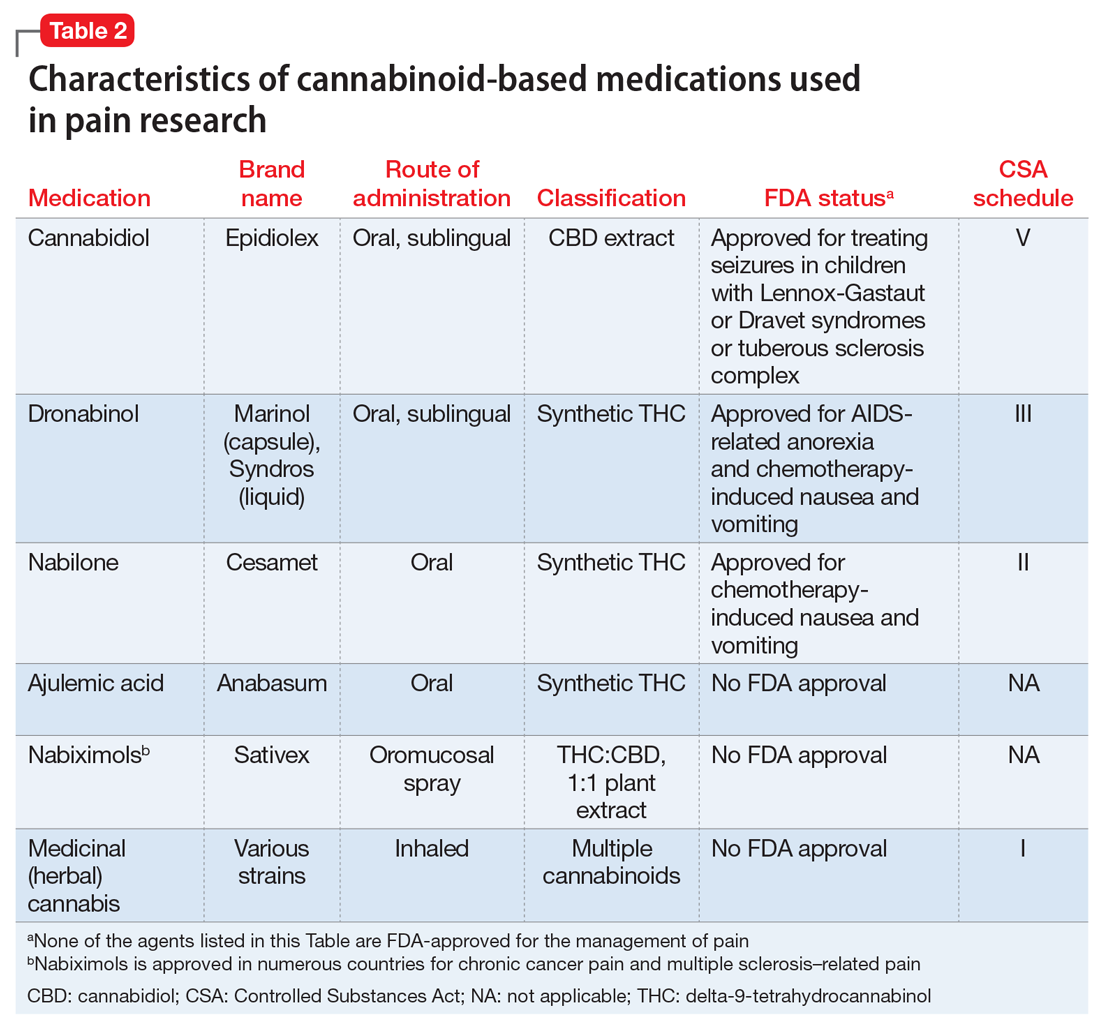
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-yRice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-xCannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws https://doi.org/10.17226/24625 http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.http://dx.doi.org/10.3747/co.23.3487