STAR*D trial. The ongoing National Institute of Mental Health (NIMH) Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial may offer a new algorithmic approach to treating major depression.14,28 NIMH launched STAR*D in 1999, enrollment began in 2001, and results are expecte by May 2005 (see Related resources).
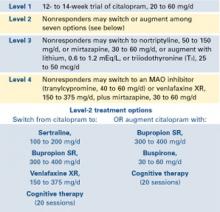
STAR*D—of which I am the study director—is a randomized, controlled, raterblinded, multisite trial of outpatients ages 18 to 75 with nonpsychotic major depression (17-item Hamilton Rating Scale for Depression score 14). The trial design includes four treatment levels and numerous antidepressant options (Figure).
The study’s aim is to enroll 4,000 patients into level 1, with 1,500 entering level 2. Patients who achieve an adequate response based on clinician judgment may continue the effective treatment for 12 months, during which their symptoms and other relevant information are monitored monthly by telephone. Patients who do not achieve an acceptable response in level 1 (or in subsequent levels) may proceed to the next level, which involves a randomized assignment.
STAR*D has an innovative design that mimics clinical practice and ensures high levels of patient participation. When patients agree to randomization, they may elect to exclude groups of treatments but may not pick a particular treatment (they must accept randomization to stay in the study).
Figure STAR*D treatment levels for major depressive disorder
For example, patient A entering level 2 may exclude switch treatments and elect to accept randomization to citalopram plus bupropion SR, citalopram plus buspirone, or citalopram and cognitive therapy. Conversely, patient B may exclude all augment options at level 2, and accept randomization to the four switch options.
Patients may exclude cognitive psychotherapy as an augment and/or switch option as long as they accept randomization to all available medication switches, or augments, or both. They may also choose cognitive therapy and exclude all medication switch and augment options. These patients must accept randomization to either cognitive therapy switch or cognitive therapy augmentation.
This so-called equipoise stratified randomized design29 allows us to compare all participants randomized to the treatments being compared. To date, only 1% of subjects have accepted randomization to all seven level-2 treatments. About one-half elect only the switch options, and about one-half elect only the augment options.
STAR*D’s goal is to determine whether there is a preferred next step for varying types and degrees of treatment-resistant repression.
Vagus nerve stimulation
Somatic therapies being investigated to expand our therapeutic options for major depressive disorder include magnetic seizure therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation (VNS).
VNS—now indicated for treatment-resistant epilepsy—is being investigated as a potential augmentation for treatment-resistant depression. An application for this supplemental indication was submitted to the FDA in October 2003.
With VNS, a device implanted in the patient’s chest provides intermittent stimulation to the left vagus nerve (typically 30 seconds on and 5 minutes off, 24 hours a day). In an open trial10 and follow-up report,30 VNS was associated with a 30% to 45% response rate in 59 depressed patients with high levels of treatment resistance (inadequate response to an average of 16 treatment trials).
VNS is well tolerated, though it has not been prospectively studied in patients with diagnosed cardiovascular disease. Side effects that may occur when the stimulation is “on” include:
- voice alteration in about 60% of patients (the voice becomes more hoarse when the left recurrent laryngeal nerve is activated)
- paresthesias in the neck
- shortness of breath on heavy exertion.
These effects are usually absent in the 5-minute “off” phase.
- National Institute of Mental Health. Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial. Includes the Quick Inventory of Depressive Symptomatology. www.star-d.org
- HealthyPlace.Com Depression Community. Vagus nerve stimulation for treating depression. www.healthyplace.com/communities/depression/treatment/vns
Drug brand names
- Bupropion • Wellbutrin, Wellbutrin SR
- Buspirone • BuSpar
- Citalopram • Celexa
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Mirtazapine • Remeron
- Modafinil • Provigil
- Nefazodone • Serzone
- Nortriptyline • Pamelor, Aventyl
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor, Effexor XR
Disclosure
Dr. Rush receives grant/research support from the Robert Wood Johnson Foundation, National Institute of Mental Health, and The Stanley Foundation. He is a consultant to Bristol-Myers Squibb Co., Cyberonics, Eli Lilly & Co., Forest Laboratories, and GlaxoSmithKline, and a speaker for Bristol-Myers Squibb Co., Cyberonics, Eli Lilly & Co., Forest Laboratories, GlaxoSmithKline, and Wyeth Pharmaceuticals.