What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
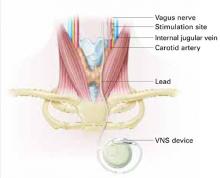
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had: