Two months ago, Mr. P, age 20, experienced paranoid thoughts, anxiety, agitation, and auditory hallucinations. During a brief hospitalization 1 month later, he received IM haloperidol, 2 mg, which he said “made his neck stiff.” After he was discharged, Mr. P, who is White, stopped taking his antipsychotics. During a recent outpatient evaluation, the clinician gives Mr. P a working diagnosis of schizophrenia and prescribes risperidone, 2 mg/d, with plans to titrate to 4 mg/d in the next 2 weeks. However, a week later, Mr. P complains of extreme sedation and feeling “knocked out” and does not want to continue taking the medication. Physical exam reveals slight cogwheel rigidity. His delusional thought content is not improved. The treating physician considers ordering a genetic test to determine Mr. P’s cytochrome P450 (CYP) 2D6 metabolizer status.
Studies investigating relationships among genetic variants thought to impact pharmacokinetics and pharmacodynamics of psychotropic medications have had mixed results.1 Metabolism of most antipsychotics depends on the CYP450 enzyme system, which is expressed predominantly in the liver (Table 1). CYP2D6 is one of these enzymes and may be responsible for metabolizing approximately 20% to 50% of all medications, including a number of antipsychotics.2 Genetic variations of CYP2D6 are common and the frequencies of these variants differ among racial groups.3
The half-life and other pharmacokinetic parameters of an antipsychotic metabolized by CYP2D6 may differ based on whether someone is a poor metabolizer (PM), intermediate metabolizer (IM), extensive metabolizer (EM), or ultrarapid metabolizer (UM).4 Regarding CYP2D6 metabolism among Whites, 3% to 5% are UMs, 70% to 80% are EMs, 10% to 17% are IMs, and 5% to 10% are PMs.5 By contrast, the percentage of PMs and UMs in the Asian population is low—about 1% for each phenotype; the IM phenotype is more common (65% to 70% in the Chinese population).5,6 The percentage of PMs in African Americans is roughly 2% to 6%.2
- Genetic variants of CYP2D6 may result in decreased or increased metabolism of some drugs, including risperidone, iloperidone, perphenazine, haloperidol, and thioridazine.
- The effect of reduced CYP2D6 activity may increase a patient’s risk for dose-related adverse effects.
- It is currently unknown if clinical genotyping for CYP2D6 variants and using this information to guide drug selection or dosing improves patient outcomes.
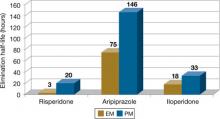
The clinical effect of altered metabolizer status depends on the extent the metabolism of a given agent is dependent on CYP2D6. PM status results in an approximately 2- to 6-fold increase in elimination half-life and overall exposure of aripiprazole,7 risperidone,8 and iloperidone9 (Figure). On the other end of the spectrum are UMs. Because of gene duplication, patients who fall into this category have enhanced metabolic activity. As a result, the therapeutic effect of several medications may be decreased because of faster clearance from the body, leading some physicians to label them as treatment-resistant.
Because side effects of many antipsychotics are dose-dependent, genotyping may be valuable for patients taking agents that are primarily metabolized by CYP2D6.10 Clinicians now have access to laboratory resources and FDA-approved methods for assessing CYP2D6 gene variants.11 It is debatable, however, whether this testing—which is expensive (≥$400) and may not be covered by health insurance—improves patient outcomes. In Mr. P’s case, if he had been genotyped as a CYP2D6 PM before treatment, his physicians might not have prescribed haloperidol and could have prevented a mild dystonic reaction. Also, they could have lowered the initial risperidone dose or chosen an antipsychotic such as ziprasidone, paliperidone, or quetiapine where the pharmacokinetic consequences of 2D6 poor metabolism are not as severe. Theoretically, one may argue that this could have reduced the risk for antipsychotic-associated side effects that now are a barrier to Mr. P’s desire to continue antipsychotics. On the other hand one may also reasonably argue that there may be other/additional reasons (genetic or non-genetic) that make some patients more sensitive to the side effects of antipsychotics and that simply assessing CYP2D6 status is not enough to guide drug selection and dosing.
Table 1
Cytochrome P450 (CYP) metabolism of commonly used antipsychotics*
| Drug | CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4/5 |
|---|---|---|---|---|---|
| Aripiprazole | X | X | |||
| Asenapine | X | X | X | ||
| Chlorpromazine | X | X | X | ||
| Clozapine | X | X | X | X | X |
| Fluphenazine | X | ||||
| Haloperidol | X | X | X | ||
| Iloperidone | X | X | |||
| Olanzapine | X | X | |||
| Paliperidone | X† | X† | |||
| Perphenazine | X | X | X | X | X |
| Quetiapine | X | X | |||
| Risperidone | X | X | |||
| Thioridazine | X | X | |||
| Ziprasidone | X | X | |||
| *Information obtained from the most recent prescribing information available from each drug’s manufacturer †According to paliperidone’s prescribing information, in vitro studies identify that CYP2D6 and CYP3A4 may be involved in paliperidone metabolism, but in vivo studies indicate that their role in eliminating paliperidone is minimal | |||||
Figure: Effects of CYP2D6 poor metabolizer status on the half-life of risperidone, aripiprazole, and iloperidone
EM: extensive metabolizer; PM: poor metabolizer
Source: References 7-9