In the United States, >50% of psychiatric inpatients have vitamin D deficiency—<30 nmol/L (<12 ng/mL).1 A growing body of literature has found associations between vitamin D deficiency and psychiatric illnesses, particularly depression. Several randomized controlled trials (RCTs) have demonstrated that vitamin D supplementation can benefit depression symptoms. In this article, we discuss the current literature on vitamin D and psychiatric illness, and provide practical information for clinicians on the use of vitamin D supplementation.
Biosynthesis of vitamin D
Biosynthesis of vitamin D begins with the sterol provitamin D3 molecule 7-dehydrocholesterol (Figure).2 When skin is exposed to sunlight, 7-dehydrocholesterol absorbs UV radiation and forms provitamin D3, which undergoes rapid transformation to vitamin D3.2 Vitamin D3 is released from the plasma membrane and enters systemic circulation in a protein-bound form that has a serum half-life of 36 to 78 hours.3 Vitamin D3 can be taken up by adipocytes and stored in fat deposits, where it has a half-life of approximately 2 months.4
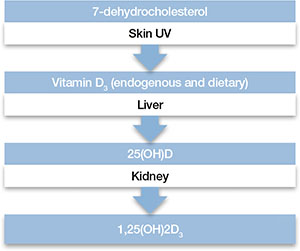
Figure: Biosynthesis of vitamin D
Provitamin D3 (7-dehydrocholesterol) in the skin absorbs UV radiation and undergoes isomerization to form vitamin D3. Endogenously produced vitamin D3 along with dietary vitamin D2 and vitamin D3 absorbed in the gastrointestinal tract are metabolized in the liver to 25-hydroxyvitamin D (25[OH]D), which re-enters the circulation and is metabolized in the kidney and other tissues to the active metabolite 1,25-dihydroxyvitamin D (1,25[OH]2D). Catabolism of 25(OH)D and 1,25(OH)2D into biologically-inactive molecules is primarily mediated by the cytochrome P450 (CYP) enzymes CYP24 and CYP3A4.
Source: Reference 2Circulating vitamin D3 is metabolized in the liver by the enzyme vitamin D-25-hydroxylase to 25-hydroxyvitamin D (25[OH]D3), which has a serum half-life of approximately 15 days.4 Circulating 25(OH)D3 is not biologically active at the physiological level, and requires activation by conversion to 1,25-dihydroxyvitamin D (1,25[OH]2D3) in the kidneys by the enzyme 25(OH)D-1α-hydroxylase. Production of 1,25(OH)2D3 is regulated by serum phosphorus and parathyroid hormone levels and other factors.5 Catabolism of 1,25(OH)2D3 is rapid, with a serum half-life of 3.5 to 21 hours.6 Vitamin D2 is structurally similar to vitamin D3, but occurs primarily in fungi, yeasts, and some invertebrates.
Risk factors for deficiency
A patient’s vitamin D status is determined by measuring 25(OH)D (Box 1). Risk factors for vitamin D deficiency include conditions that affect cutaneous production (insufficient sunlight exposure), obesity, gastrointestinal disorders, aging, renal disorders, and medications (Table 1). 2,5,7,8 The link between sunscreen use, either alone or in cosmetics, and vitamin D deficiency continues to be debated. While controlled studies have found that application of sunscreen with high sun protection factor can significantly reduce vitamin D production, 9 studies in clinical populations have failed to confirm these findings. 10,11 See Box 2 for a discussion of these risk factors and Box 3 for a discussion of acute and long-term medical manifestations of deficiency.
Although 1,25-dihydroxyvitamin D (1,25[OH]2D3) is the biologically active form of vitamin D, its circulating half-life is only 4 to 6 hours.a,b Therefore, 25-hydroxyvitamin D (25[OH]D) is the principal vitamin D metabolite measured to determine vitamin D status. Vitamin D levels commonly are expressed as ng/mL or nmol/L; the conversion factor from ng/mL to nmol/L is 2.496. The Institute of Medicine has defined vitamin D deficiency as a serum 25(OH)D level of <30 nmol/L (<12 ng/mL).c However, many experts define vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/ml, and deficiency as <20 ng/mL.a,d The upper limit is more difficult to define, but symptoms of vitamin D intoxication appear with blood levels >150 to 200 ng/mL.a
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Aloia JF. Clinical review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;96(10):2987-2996.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
Table 1
Risk factors associated with vitamin D deficiency
| Age (>65) |
| Insufficient sunlight |
| Breastfeeding |
| Dark skin |
| Malabsorption diseases |
| Obesity (BMI >30 kg/m2) |
| Use of medications that alter vitamin D metabolism (eg, anticonvulsants, glucocorticoids) |
| Hepatobiliary disease |
| Renal disease |
| BMI: body mass index Source: References 2,5,7,8 |
Any factor that diminishes UV radiation penetration into the skin will affect cutaneous synthesis of vitamin D.a,b For example, sunscreen with a sun protection factor of 15 can decrease vitamin D synthesis by 98%.c Geography and its impact on yearly sunlight exposure is a well-known factor in vitamin D deficiency. Individuals who live below a latitude of approximately 35° North—approximately the southern border of Tennessee and through Albuquerque, NM—receive sufficient UV radiation exposure to ensure adequate vitamin D production throughout the year, but at higher latitudes, adequate vitamin D is not produced during winter months.d Melanin affects UV radiation absorption in a manner that prevents vitamin D production, and increased skin pigmentation markedly reduces vitamin D synthesis.e African Americans with very dark skin have significantly diminished cutaneous production of vitamin D.e,f
Renal 1α-hydroxylase activity decreases with aging in parallel with age-related decreases in glomerular filtration.g In addition, aging is associated with increased clearance of 1,25-dihydroxyvitamin D (1,25[OH]2D3).h However, vitamin D absorption generally is adequate even at older ages.i Studies have shown that obese individuals tend to have lower serum concentrations of vitamin D and 25-hydroxyvitamin D (25[OH]D) than those at a normal weight.j,k Obese patients have been shown to have lower cutaneous production of vitamin D3 and display lower bioavailability of orally administered vitamin D2.j
For patients with chronic renal insufficiency, creatinine clearance is positively correlated with serum 1,25(OH)2D levels.l Any process that results in malabsorption of intestinal fat may impair vitamin D absorption. In patients with celiac disease, biliary obstruction, or chronic pancreatitis, absorption consistently is reduced.m Individuals taking bile acid-binding medications, such as cholestyramine for hypercholesterolemia, also may have impaired vitamin D absorption.n In addition, hepatobiliary disease is associated with low levels of 25(OH)D.o Some drugs that alter hepatic metabolism are associated with vitamin D deficiency, including anticonvulsants or glucocorticoids, which can increase catabolism or vitamin D.p
References
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29(6):361-368.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
- Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
- Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213-217.
- Slovik DM, Adams JS, Neer RM, et al. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305(7):372-374.
- Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J Clin Invest. 1980;66(5):1118-1123.
- Lips P, Wiersinga A, van Ginkel FC, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644-650.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
- Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370-373.
- Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab. 1988;67(5):876-881.
- Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45(1):94-102.
- Lo CW, Paris PW, Clemens TL, et al. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644-649.
- Pappa HM, Bern E, Kamin D, et al. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24(2):176-183.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.

