Mr. H, age 31, is admitted to an acute psychiatric unit with major depressive disorder, substance dependence, insomnia, and generalized anxiety. In the past, he was treated unsuccessfully with sertraline, fluoxetine, clonazepam, venlafaxine, and lithium. The treatment team starts Mr. H on quetiapine, titrated to 150 mg at bedtime, to address suspected bipolar II disorder.
At baseline, Mr. H is 68 inches tall and slightly overweight at 176 lbs (body mass index [BMI] 26.8 kg/m2). The laboratory reports his glycated hemoglobin (HbA1c) at 5.4%; low-density lipoprotein (LDL), 60 mg/dL; total cholesterol, 122 mg/dL; triglycerides, 141 mg/dL; and high-density lipoprotein (HDL), 34 mg/dL.
Within 1 month, Mr. H experiences a 16% increase in body weight. HbA1c increases to 5.6%; LDL, to 93 mg/dL. These metabolic changes are not addressed, and he continues quetiapine for another 5 months. At the end of 6 months, Mr. H weighs 223.8 lbs (BMI 34 kg/m2)—a 27% increase from baseline. HbA1c is in the prediabetic range, at 5.9%, and LDL is 120 mg/dL.1 The treatment team discusses the risks of further metabolic effects, cardiovascular disease, and diabetes with Mr. H. He agrees to a change in therapy.
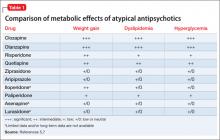
An increase in weight is thought to be associated with the actions of antipsychotics on H1and 5-HT2c receptors.7 Clozapine and olanzapine pose the highest risk of weight gain. Quetiapine and risperidone are considered of intermediate risk; aripiprazole and ziprasidone present the lowest risk(Table 1).5,7
Patients taking an atypical antipsychotic may experience an elevation of blood glucose, serum
• All atypical antipsychotics carry a risk of metabolic disturbance; clozapine and olanzapine have the highest risk, followed by quetiapine and risperidone.
• Newer atypical antipsychotics may carry less of a risk of metabolic side effects, but long-term data are lacking.
• Obtain baseline and periodic monitoring of BMI, waist circumference, HbA1c, fasting plasma glucose, and fasting lipids.
• If you find an abnormality of any of these parameters, consider one or more of the following: switching to an agent that is less risky; decreasing the dose or discontinuing therapy; recommending diet and exercise; and referring the patient to a program or clinician with expertise in the management of weight, diabetes, or lipids.
• Use monotherapy when appropriate to decrease the risk of side effects.triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic â-cells.7 Clozapine and olanzapine pose the highest risk of dyslipidemia. Quetiapine and risperidone are considered of intermediate risk; the risk associated with quetiapine is closer to that of olanzpine.8,9 Aripiprazole and ziprasidone present a lower risk of dyslipidemia and glucose elevations.5
Newer atypical antipsychotics, such as asenapine, iloperidone, paliperidone, and lurasidone, seem to have a lower metabolic risk profile, similar to those seen with aripiprazole and ziprasidone.5 Patients enrolled in initial clinical trials might not be antipsychotic naïve, however, and may have been taking a high metabolic risk antipsychotic. When these patients are switched to an antipsychotic that carries less of a metabolic risk, it might appear that they are experiencing a decrease in metabolic adverse events.
Metabolic data on newer atypical antipsychotics are limited; most have not been subject to long-term study. Routine monitoring of metabolic side effects is recommended for all atypical antipsychotics, regardless of risk profile.
Recommended monitoring
Because of the known metabolic side effects that occur in patients taking an atypical antipsychotic, baseline and periodic monitoring is recommended (Table 2).2,10 BMI and waist circumference should be recorded at baseline and tracked throughout treatment. Ideally, obtain measurements monthly for the first 3 months of therapy, or after any medication adjustments, then at 6 months, and annually thereafter. Encourage patients to track their own weight.