Wound complications (eg, delayed wound healing, blisters, prolonged drainage) have been reported in up to 30% of patients who undergo elective total joint arthroplasty (TJA).1-6 Wound complications increase resource utilization, lengthen hospital stays, and increase costs.7-9 Prolonged wound healing and persistent wound drainage are also harbingers of both superficial and deep surgical site infections.5-11
In several studies, wound complications after TJA were the primary reason for hospital readmissions.12-15 As part of the Patient Protection and Affordable Care Act, hospitals will be penalized by the Centers for Medicaid & Medicare Services for unplanned hospital readmissions within 30 days after TJA. It is imperative, then, to reduce the risk factors and complications associated with surgical site infections to decrease unplanned readmissions.
Historically, little attention has been given to the role of surgical dressings and the effect of dressings on wound healing. Although many subspecialties (eg, cardiothoracic surgery, general surgery) have reported benefits in using occlusive dressings, adoption in TJA has been slow.16-18 At our institution about 5 years ago, we began using an occlusive silver-impregnated barrier dressing based on preliminary data from studies showing benefits of occlusive dressings in TJA.19,20
We conducted a study to determine if use of occlusive antimicrobial barrier dressings decreases rates of wound complications in TJA. We had 3 research questions: Compared with standard surgical dressings, are occlusive dressings associated with decreased rates of wound complications after TJA? Is there a difference in number of dressing changes required between the 2 dressing types? Is satisfaction higher for patients with occlusive dressings than for patients with standard dressings?
Patients and Methods
This randomized controlled trial (RCT) was reviewed and approved by the Institutional Review Board at Carolinas Healthcare. Patients were randomized by the research staff using a parallel, 1:1 allocation method. The randomization table was generated using a random number generator.
An a priori sample size estimate was made using a 2-tailed Fisher exact test with a .05 level of significance. Based on a study by Clarke and colleagues,21 we estimated the incidence of wound problems at 3% in the occlusive dressing (study) group and 13% in the standard dressing (control) group. We determined that 260 participants (130 per group) would be needed to achieve 80% power. We considered a 15% attrition rate for a total enrollment goal of 300 study participants (150 per group).
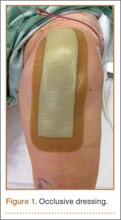
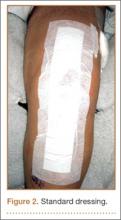
Between December 2010 and January 2013, patients presenting for either primary total hip arthroplasty (THA) or primary total knee arthroplasty (TKA) were recruited to participate in the study. Eligibility criteria (Table 1) were reviewed, and patients were enrolled by the senior surgeons, Dr. Springer, Dr. Beaver, Dr. Griffin, and Dr. Mason. All eligible participants who provided informed consent were randomized to receive either an occlusive antimicrobial barrier dressing (Aquacel Ag, ConvaTec) or standard surgical dressing (Primapore, Smith & Nephew). The occlusive dressing (Figure 1) consists of an outer barrier layer of hydrocolloid and a central island of hydrofiber, which absorbs and locks in any wound exudate within the fibers and prevents the creation of an overly moist wound environment that can lead to skin maceration and wound breakdown. In addition, the hydrofibers are embedded with ionic silver, which is released only at the site of wound exudate, or drainage; thus, there is no continuous exposure of the entire wound to silver. The standard dressing (Figure 2) consists of a central island of gauze enclosed in low-allergy acrylic adhesive tape.
All surgical dressings were placed over a closed incision in a sterile environment in the operating room after the procedure. The groups’ wound closures were identical.
A posterior approach was used for all THAs. The deep fascia was closed with a running barbed suture (Quill, Angiotech), the deep subcutaneous tissue with No. 1 Vicryl suture (Ethicon), and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch (Ethicon) was placed in the subcuticular layer and was followed with a skin adhesive (Dermabond, Ethicon). A closed suction drain, removed on postoperative day (POD) 1, was used for all THAs.
A standard medial parapatellar arthrotomy was used for all TKAs. The arthrotomy was closed with a running barbed suture, the deep subcutaneous tissue with No. 1 Vicryl suture, and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch was placed in the subcuticular layer and was followed with a skin adhesive. A closed suction drain was also used. In addition, a compressive wrap was placed over the dressing in the operating room and was removed the next morning. During the hospital stay, the surgical site was evaluated daily with a standard wound evaluation form.