Anterior cruciate ligament (ACL) reconstruction remains one of the most common orthopedic procedures; almost 100,000 are performed in the United States each year, and they are among the procedures more commonly performed by surgeons specializing in sports medicine and by general orthopedists.1,2 Recent years have seen a trend toward replacing the gold standard of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.3 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, graft fixation strength has become a priority in attempts to optimize recovery and rehabilitation.4
Rigid fixation of hamstring grafts is now recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand both early rehabilitation forces as high as 500 N5 and stresses to the native ACL during healing, which may take up to 12 weeks for soft-tissue incorporation.6
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed, and there is controversy regarding which provides the best stability and strength.7 Several studies have tested various fixation devices,8-16 but so far several devices have not been compared with one another.
We conducted a study to determine if femoral hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Fifty porcine femurs were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
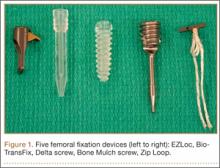
We evaluated 5 different femoral fixation devices (Figure 1): Delta screw and Bio-TransFix (Arthrex, Naples, Florida) and Bone Mulch screw, EZLoc, and Zip Loop (Arthrotek, Warsaw, Indiana). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the femurs using the 5 femoral fixation devices. All fixations were done to manufacturer specifications.
Cyclic loading was followed by testing with the load-to-failure (LTF) protocol described by Kousa and colleagues.13 Specimens were tested in a custom load fixture (Figure 2). The base fixture used an adjustable angle vise mounted on a free rotary stage and a free x-y translation stage. This system allowed the load axis to be oriented to and aligned with the graft tunnel in the porcine femur, preventing off-axis or torsional loading of the grafts.
Pneumatic grips equipped with a custom pincer attachment allowed the graft to be grasped under a constant grip force during testing, regardless of graft thinning under tensile loads. Graft specimens were initially looped over a 3.8-mm horizontal metal shaft, and the 2 strands were double-looped at the graft insertion site. The 2 free strands were then drawn up around the metal shaft, and the shaft was placed above the serrated jaws. The metal shaft with enveloping tendon strands rested on a flat shelf at the top of the grip serrations. This configuration prevented the metal shaft and tendon strands from being pulled through the serrations when compressive force was applied to the jaws.
Before the study, the grip design was tested. There was no detectable relative motion of the strands at the grip end during graft testing to failure. The pincer attachment allowed close approach of the grips to the specimen at all femoral condyle orientations, so that a 25-mm length of exposed graft could be obtained for each specimen under initial conditions.
In the cyclic loading test, the load was applied parallel to the long axis of the femoral tunnel. A 50-N preload was initially applied to each specimen for 10 seconds, and the length of the exposed graft between grips and graft insertion was recorded. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 2 seconds (0.5 Hz) were performed. Standard force-displacement curves were then generated.
Specimens surviving the cyclic loading then underwent a single-cycle LTF test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF data were generated from the single-cycle LTF test; ultimate LTF was defined as the maximum load sustained by the specimen during a constant-displacement-rate tensile test for graft pullout.