Letter to the Editor
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
We read with great interest the study by Nodzo and colleagues in the May 2014 issue of The American Journal of Orthopedics on hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection.1 We agree with the authors that determining if a P acnes culture is a true infection or a contaminant remains a challenge. Although P acnes is described as a commensal bacterium with a low pathogenicity, its involvement has been reported in many clinical entities, especially device-related infections.2P acnes is usually the cause of delayed infections occurring 3 to 24 months or more after prosthesis placement. The rate of P acnes involvement, probably underestimated, is about 10%.3 Although this bacterium was considered to be a contaminant, several virulence factors have been recently identified: putative hemolysins or cytotoxins (CAMP factors, hemolysin III) and enzymes putatively involved in degrading host tissue or molecules (GehA lipase, lysophospholipase, hyaluronate lyase, endoglycoceramidase, etc).4
Interestingly, Nodzo and colleagues revealed that 13 out of 22 P acnes strains were hemolytic and, among them, 10 were considered as definite infections, including 3 with only 1 positive sample. The authors could not identify a statistically significant trend, probably because their study was underpowered due to the size of this case series, as discussed by the authors. Nevertheless, the hemolytic activity of the strains was investigated in the 1980s by adding different concentrations of blood obtained from rabbits, sheep, or humans.5 The hemolytic activity was recorded as positive when a clear, colorless zone around the colonies appeared or weak when slight and incomplete hemolysis under the colonies was found.5 Depending on the erythrocyte origin, differences in the lytic action of hemolysin or cytotoxin may indicate the existence of various enzymes. These enzymes could have different levels of production and provide a distinct hemolytic profile. This hemolytic activity observation could also be correlated to the genetic background of the isolates.
In fact, from a genetic and epidemiological point of view, the sequence analysis of recA gene distinguished 2 distinct lineages of P acnes: types I and II.4 The association of some strains with specific clinical presentations was also demonstrated. Later, McDowell and colleagues6 reported 5 main phylogenetically distinct groups: IA, IB, IC, II, and III. It would have been interesting to know the phylogenetic groups of the strains tested in the study by Nodzo and coauthors, especially as Sampedro and colleagues7 recently reported more phylogenetic groups IA and IB among P acnes strains involved in bone and joint infections. Both of these phylotypes are hemolytic, unlike phylotypes II and III, less often encountered in this clinical entity as reported recently.8 We agree with the authors that hemolytic behavior may be one of the key factors in the variability in the pathogenicity of P acnes strains, suggesting that some strains could be more aggressive than others during deep infection. Another feature is likely the biofilm-production ability of the strains.9,10
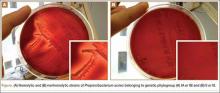
According to our experience, the hemolysis behavior was slightly different depending on which blood agar plates were used to detect hemolytic properties. We have selected 8 isolates or reference ATCC strains from different phylotypes. Each isolate was seeded on 5 different blood agar plates with erythrocyte from various origins (Table). We can confirm that only strains belonging to IA and IB phylotypes were hemolytic, with different behavior as previously reported (Figure).8 Similarly, within IA phylotype strains, the hemolytic property could be different suggesting a difference in the genetic background. However, as the genes encoding all 5 CAMP factors are present in all P acnes groups studied by Valanne and colleagues11 (IA, IB, and II), observed differences reflected different levels of expression rather than missing genes. Moreover, when camp2 or camp4 genes were deleted, the ∆camp2 but not the ∆camp4 mutant exhibited reduced hemolytic activity with sheep erythrocytes, indicating that CAMP factor 2 seems to be the major active cohemolytic factor, but in an IA phylotype P acnes genetic background.12
To conclude, the link between hemolysis and P acnes deep infection remains controversial and complex. The phenotypic differences observed between strains from various types reflect deeper differences in their phylogeny. The hemolytic ability raises the possibility that strains may also display a specific behavior according to their type and variation in their expression of putative virulence factors, including hemolysin, cytotoxin, or lipase. Further studies are clearly needed to better understand the virulence and phylogeny of P acnes strains in order to distinguish contamination from bone infection.