From the Cosmetic Dermatology Archives
Cosmetic Corner: Dermatologists Weigh in on Face Washes
Leading dermatologists offered their recommendations on the top face washes. See what they’re recommending to their patients and why.
Dr. Draelos is from Dermatology Consulting Services, High Point, North Carolina. Dr. Fowler is from Dermatology Specialists, PSC, Louisville, Kentucky. Dr. Larsen is from Portland Dermatology Clinic, Oregon. Ms. Hornby, Dr. Walters, and Dr. Appa are from Johnson & Johnson Consumer Inc, Skillman, New Jersey.
These studies were supported by Johnson & Johnson Consumer Inc. Dr. Draelos received a research grant from Johnson & Johnson Consumer Inc. Dr. Fowler has served on the advisory board for and has received research grants from Johnson & Johnson Consumer Inc. Dr. Larsen reports no conflict of interest. Ms. Hornby, Dr. Walters, and Dr. Appa are or were employees of Johnson & Johnson Consumer Inc at the time these studies were carried out. Dr. Walters also is an inventor on patents owned by Johnson & Johnson Consumer Inc.
Correspondence: Sidney Hornby, MS, Johnson & Johnson Consumer Inc, 199 Grandview Rd, Skillman, NJ 08558 (shornby@its.jnj.com).

Participants were instructed to wash their face twice daily, noting the time of cleansing and providing commentary about their cleansing experience in a diary. The liquid facial test cleansers contained the HMP potassium acrylates copolymer, glycerin, and a surfactant system primarily containing cocamidopropyl betaine and lauryl glucoside prepared without added fragrance (as previously described20) or with a fragrance free of common allergens and irritating essential oils.
Half of the participants used the fragranced test cleanser and half used the fragrance-free test cleanser for a 3-week treatment period (weeks 1–3). Each treatment group subsequently switched to the other test cleanser for a second 3-week treatment period (weeks 4–6). Clinicians assessed global disease severity (an overall assessment of skin condition that was independent of other evaluation criteria), itching/burning, visible irritation, erythema, and desquamation at weekly time points throughout the study and graded each clinical tolerance attribute on a 5-point scale (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe). Ordinal scores at baseline and at weeks 1 and 3 were used to calculate change from baseline.
A 7-item questionnaire also was administered to participants at each visit to assess skin condition, smoothness, softness, cleanliness, radiance, satisfaction with cleansing experience, and lathering. Each item was scored on a 5-point ordinal scale (0=none; 1=minimal; 2=good; 3=excellent; 4=superior). The scores for all parameters were statistically compared with baseline values using a paired t test with a significance level of P≤.05.
Study 2 Design
This prospective, 3-week, double-blind, randomized, comparative, 2-center study to evaluate the tolerability of the fragranced, HMP-containing test cleanser from study 1 versus a benchmark gentle, fragrance-free, nonfoaming cleanser in a large population of otherwise healthy females who had been clinically diagnosed with sensitive skin (not limited to fragrance sensitivity). The study sponsor provided blinded test materials, and neither the examiner nor the recorder knew which investigational product was administered to which participants. Additionally, personnel who dispensed the test cleansers to participants or supervised their use did not participate in the evaluation to minimize potential bias. All participants provided written informed consent prior to enrolling in the study, and the study protocol and informed consent agreement were approved by an institutional review board.
Participants included women aged 18 to 65 years with mild to moderate clinical symptoms of atopic dermatitis, eczema, acne, or rosacea within the 90 days prior to the study period. They were randomized into 2 balanced treatment groups: group 1 received the mild, fragranced, HMP-containing liquid facial cleanser from study 1 and group 2 received a leading, dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser. Each treatment group used the test cleansers at least once daily for 3 weeks.
Clinicians evaluated facial skin for softness and smoothness, global disease severity (rated visually by the investigator as an overall assessment of skin condition that was independent of other evaluation criteria [as previously described20]), itching, irritation, erythema, and desquamation at baseline and at weeks 1 and 3. The effectiveness of each product to remove facial dirt, cosmetics, and sebum also was assessed; clinical grading was performed as described for study 1 using the same grading scale as in study 1 and percentage change from baseline (improvement) was calculated.
The study also included a self-assessment of skin irritation in which participants responded yes or no to the following question: Have you experienced irritation using this product? Participants also completed a questionnaire in which they were asked to select the most appropriate answer—agree strongly, agree somewhat, neither, disagree somewhat, and disagree strongly— to the following statements: the cleanser leaves no residue; cleanses deep to remove dirt, oil, and makeup; the cleanser effectively removes makeup; the cleanser leaves my skin smooth; the cleanser leaves my skin soft; the cleanser rinses completely clean; cleanser does not over dry my skin; and my skin is completely clean.
The statistical analysis was performed using a nonparametric, 2-tailed, paired Mann-Whitney U test, and statistical significance was set at P≤.05.
Results
Study 1 Assessment
Eight female participants aged 22 to 60 years with clinically diagnosed fragrance sensitivity were enrolled in the study. After 3 weeks of use, clinician assessment showed that both the fragranced and fragrance-free test cleansers with HMPs improved several skin tolerance attributes, including global disease severity, irritation, and erythema (Figure 1). No notable differences in skin tolerance attributes were reported in the fragranced versus the fragrance-free formulations.
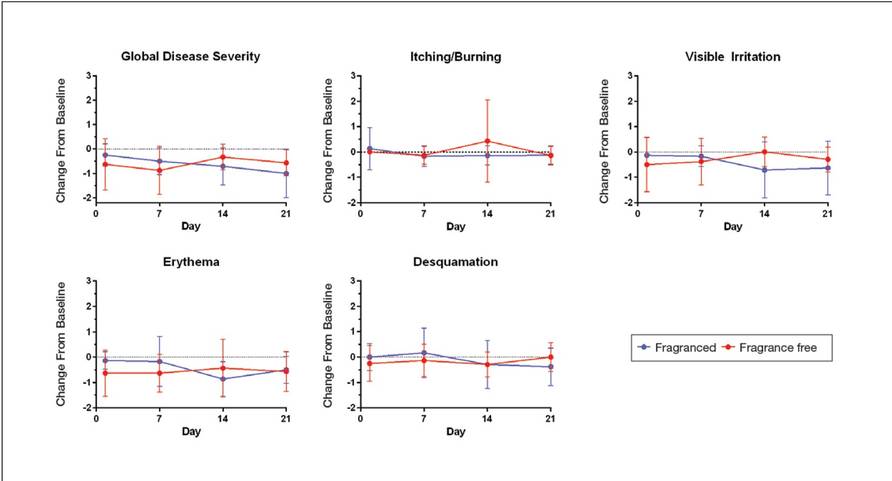
Figure 1. Investigator evaluation of skin tolerance to fragranced and fragrance-free cleansers containing hydrophobically modified polymers after 3 weeks of treatment. Mean reduction from pretreatment baseline score signifies improvement. Error bars indicate standard deviation. Tolerance attributes were scored on a 5-point scale (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe).
Leading dermatologists offered their recommendations on the top face washes. See what they’re recommending to their patients and why.

Leading dermatologists offered their recommendations on the top face washes. See what they’re recommending to their patients and why.
