Results
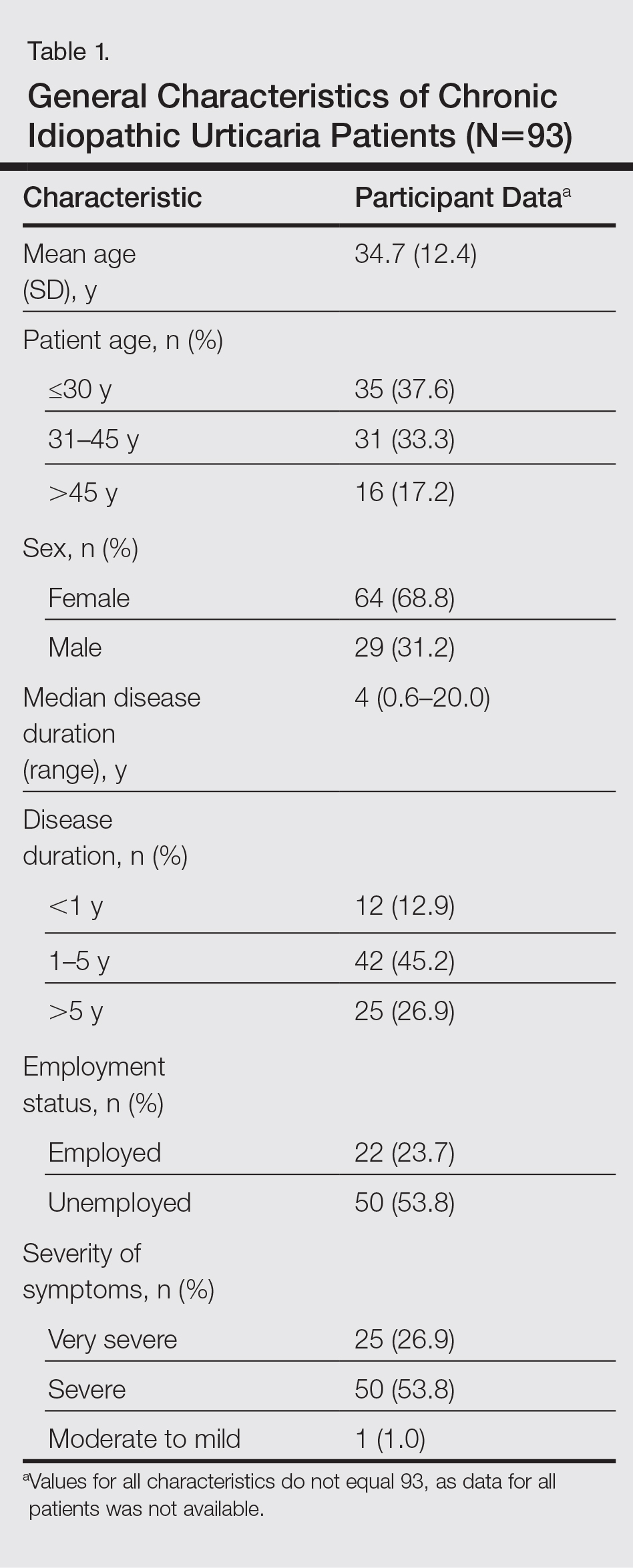
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).
The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
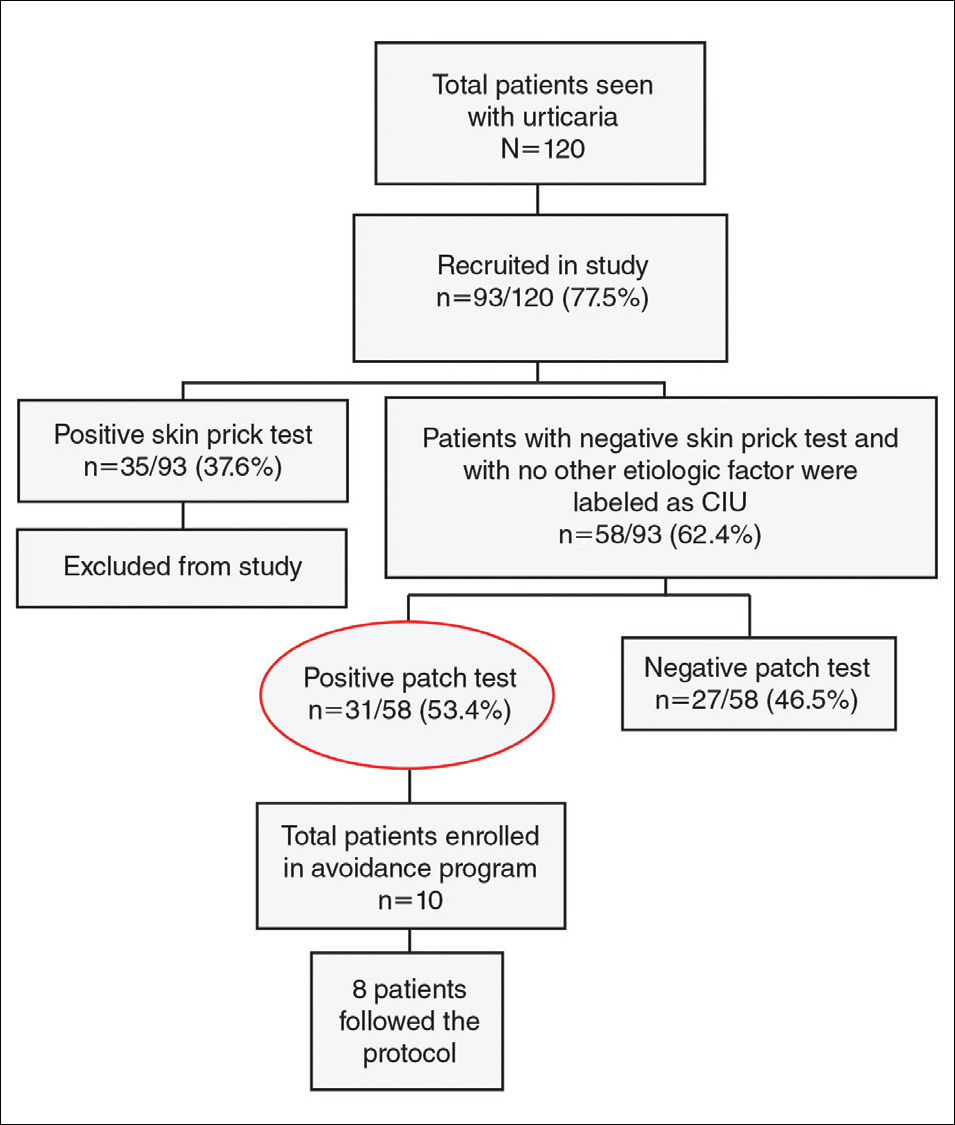
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).
Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). Twenty of 31 (64.5%) patch test–positive patients were aged 30 to 45 years. Positive patch test results were observed in 31 of 43 female patients (72.1%; P<.001). Of the patch test–positive patients, disease duration was greater than 5 years in 16 of 31 patients (51.6%).
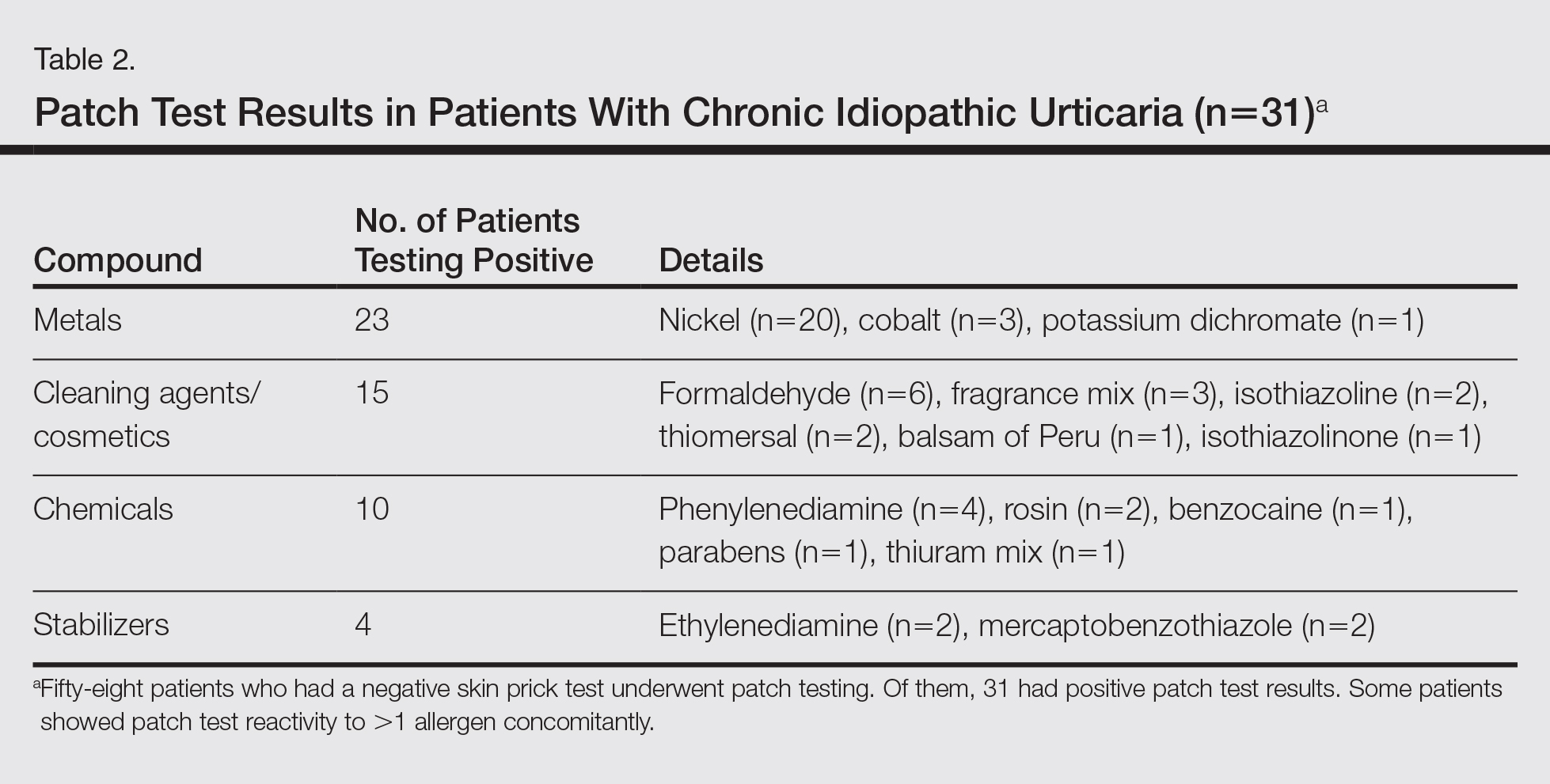
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).
Of the 31 patch test–positive patients, 10 were enrolled but only 8 (25.8%) agreed to take appropriate avoidance measures for the sensitizing substances; 5 (62.5%) showed excellent improvement in their baseline symptoms at a 1-month follow-up visit.
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The efficacy of performing patch tests in CIU has not yet been proven, as there are conflicting results regarding the role of contact sensitization in CIU. Prior studies in this field have shown that contact allergy can play a role in the etiopathogenesis of CU; these findings have stimulated new approaches for investigation of CIU.8,12 There were no details of how a common allergen such as nickel was avoided, which caused remission in the majority of patch test–positive patients.
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The role of contact sensitization in CU has not yet been established, as another study showed no relationship between avoidance of contact allergens and the course of CIU.25 In that study, patients with severe CIU who previously had been patch tested were retrospectively studied. Three groups were studied: CIU patients with positive patch tests; CIU patients with negative patch tests; and a control group, which included patients with CIU who had not been patch tested. The groups were followed monthly to assess changes in CUSS after allergen avoidance. Forty-three patients with severe CIU were patch tested. Nickel sulfate testing was positive in 4 cases (9.3%); potassium dichromate testing was positive in 2 cases (4.7%); and cobalt, balsam of Peru, paraphenylenediamine, fragrance mix, and epoxy resin testing were positive in 1 case (2.3%) each. The mean (SD) baseline CUSS score (5.4 [0.5]) significantly improved after 1 month of allergen avoidance (3.2 [1.1]; P<.001); however, similar improvement in CUSS was observed in 34 patients with CIU with negative patch test results (5.3 [0.5] to 3.2 [1.3]; P<.001) and in 49 patients with CIU in the control group after 1 month (5.2 [0.4] to 3.4 [1.3]; P<.001).25
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.