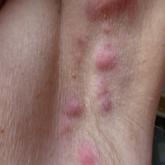
We applaud Kimball et al1 on their report that adalimumab demonstrated clinical improvement in patients with hidradenitis suppurativa (HS) versus placebo in 2 phase 3 trials. Hidradenitis suppurativa is a chronic relapsing condition with painful subcutaneous abscesses, malodorous drainage, sinus tract formation, and scarring that typically occurs in the axillae and anogenital region. It impairs the quality of life for these patients, as evidenced by higher Dermatology Life Quality Index scores compared to psoriasis, pimples, hand rash, atopic eczema, or control.2
The exact pathogenesis of HS is unknown but likely involves a complex interaction of genetic, hormonal, immunologic, and environmental factors.3 The levels of inflammatory cytokines are elevated in HS lesions, specifically IL-1β, tumor necrosis factor α, IL-10, and CXCL9, as well as monokines from IFN-γ, IL-11, and IL-17A. Additionally, the dermis of affected regions contains IL-12– and IL-23–containing macrophages along with IL-17–producing T cells.3 These findings reveal many potential therapeutic targets for the treatment of HS.
PIONEER I and PIONEER II are similarly designed 36-week phase 3 trials of 633 patients with HS who were unresponsive to oral antibiotic treatment.1 By week 12, a significantly greater proportion of patients receiving adalimumab demonstrated clinical improvement (≥50% reduction in total abscess and nodule count) compared to placebo in both trials (PIONEER I: 41.8% vs 26.0%, P=.003; PIONEER II: 58.9% vs 27.6%, P<.001). Secondary end points (inflammatory-nodule count, pain score, and disease severity) were only achieved in PIONEER II. The difference in clinical improvement between the trials is likely due to higher baseline disease severity in the HS patients in PIONEER I versus PIONEER II. No new safety risks were reported and were in accordance with prior adalimumab trials for other diseases. Notably, 10 paradoxical psoriasislike eruptions were reported.1
Adalimumab is the first and only US Food and Drug Administration–approved therapy for HS. Further understanding of the pathogenesis of HS may result in additional biologic treatments for HS. We encourage the manufacturers of other biologic therapies, such as infliximab,4 ustekinumab,5 anakinra,6 secukinumab, ixekizumab, and brodalumab, to consider conducting further clinical trials in HS to enhance the therapeutic options available for this debilitating disease.