Dermpath Diagnosis
Pretibial Myxedema
Pretibial myxedema is a cutaneous mucinosis associated with thyroid dysfunction. The differential diagnosis includes nephrogenic systemic fibrosis...
Anthony J. Kruse, BA; Claudia I. Vidal, MD, PhD
From the Department of Dermatology, Saint Louis University, Missouri.
The authors report no conflict of interest.
Correspondence: Claudia I. Vidal, MD, PhD, Department of Dermatology, Anheuser-Busch Eye Institute, 4th Floor, Room 402,
1755 S Grand Blvd, St. Louis, MO 63104 (cvidal1@slu.edu).

Subcutaneous panniculitislike T-cell lymphoma is a cutaneous lymphoma of α and β phenotype cytotoxic T cells in which the neoplastic cells are found almost exclusively in the subcutaneous layer and resemble a panniculitis. The differential diagnosis includes α1-antitrypsin deficiency panniculitis, erythema induratum, infectious panniculitis, and lupus erythematosus panniculitis.
Subcutaneous panniculitislike T-cell lymphoma (SPTL) is a cutaneous lymphoma of α and β phenotype cytotoxic T cells in which the neoplastic cells are found almost exclusively in the subcutaneous layer and resemble a panniculitis.1 It affects males and females with equal incidence and is seen in both adults and children. Clinically, this disease presents as a nonspecific panniculitis with indurated but typically nonulcerated erythematous plaques and nodules most commonly located on the extremities. Plaques and nodules may appear on other body sites and may be generalized.1 In some cases, patients present with associated systemic symptoms including fever, malaise, weight loss, and fatigue.2
Histologically, SPTL presents as a predominantly lobular panniculitis (Figure 1) with rimming of adipocytes by neoplastic cells that appear as small and medium-sized atypical lymphocytes with hyperchromatic nuclei (Figure 2A). A less dominant septal component may be present, and neoplastic cells may encroach into the lower reticular dermis, rarely involving the papillary dermis or epidermis.2 Although rimming of adipocytes is classic, it is not specific to this entity, as rimming also can be found in other lymphomas and infectious panniculitis. Reactive lymphocytes and macrophages with ingested lipid material also are seen intermixed with neoplastic cells.2 Necrosis is a common finding, including destructive fragmentation of the nucleus, known as karyorrhexis. If necrosis is extensive, appreciation of other histologic features may be hindered.3 Histiocytes engulfing the nuclear debris known as beanbag cells also can be seen (Figure 2B). The diagnosis can be made on immunohistologic analysis demonstrating neoplastic cells with a cytotoxic α and β T-suppressor phenotype centered around and rimming the adipocytes in the subcutaneous fat.3 Immunohistochemistry reveals positive CD3, CD8 (Figure 2C), and βF1 markers, as well as T-cell intracellular antigen 1 (TIA-1), granzyme B, and perforin.1,2 The neoplastic cells often have a high proliferation index as evidenced by MIB-1 (Ki-67) labeling (Figure 2D). The neoplastic cells are negative for CD4, CD56, and CD30.1,2 Subcutaneous panniculitislike T-cell lymphoma cells are negative for Epstein-Barr virus by in situ hybridization.2
| Figure 1. Subcutaneous panniculitislike T-cell lymphoma showing a predominantly lobular panniculitis (H&E, original magnification ×20). |
| Figure 2. Rimming of adipocytes by hyperchromatic lymphocytes (A)(H&E, original magnification ×400). Arrowhead indicates a histiocyte (ie, beanbag cell) that has undergone cytophagocytosis of nuclear debris (B)(H&E, original magnification ×600). Immunohistochemistry with CD8 highlights the cells rimming the adipocytes (C)(original magnification ×600). Immunohistochemistry with MIB-1 shows an increased proliferative rate in the lymphocytes rimming the adipocytes (D)(original magnification ×600). |
Subcutaneous panniculitislike T-cell lymphoma must be distinguished from lupus erythematosus panniculitis (LEP) and other cutaneous lymphomas. Importantly, LEP and SPTL clinically may appear similar and are not mutually exclusive diagnoses.2 On histology, they may look similar, showing T cell aggregates and necrosis; however, thickening of the basement membrane, vacuolar change at the dermoepidermal junction, plasma cells, hyaline sclerosis, mucin deposition, a lymphocytic perivascular infiltrate, and nodular aggregates of B cells are more common in LEP (Figure 3).2,4 Additionally, in LEP the T cell aggregates typically will not have a high proliferative rate as evidenced by MIB-1.3
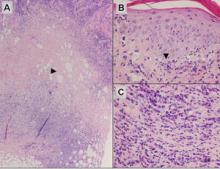 Figure 3. Lupus erythematosus panniculitis showing a lobular panniculitis with concomitant septal panniculi-tis (A)(H&E, original magnification ×40). Arrowhead indicates an area of hyaline sclerosis. Epidermal changes, including an interface dermatitis shown by the arrowhead, can be seen in up to half of cases (B)(H&E, original magnification ×400). Plasma cells may be a helpful clue in the diagnosis of lupus erythematosus panniculitis (C)(H&E, original magnification ×400).
Figure 3. Lupus erythematosus panniculitis showing a lobular panniculitis with concomitant septal panniculi-tis (A)(H&E, original magnification ×40). Arrowhead indicates an area of hyaline sclerosis. Epidermal changes, including an interface dermatitis shown by the arrowhead, can be seen in up to half of cases (B)(H&E, original magnification ×400). Plasma cells may be a helpful clue in the diagnosis of lupus erythematosus panniculitis (C)(H&E, original magnification ×400).
Additionally, other lobular panniculitides can be considered in the differential diagnosis, including erythema induratum (EI), α1-antitrypsin deficiency panniculitis (A1ATDP), and infectious panniculitis. Histologically, EI (Figure 4), also known as nodular vasculitis when not associated with Mycobacterium tuberculosis, has a lobular pattern of inflammation. Early in the disease process there are discrete collections of neutrophils; later, granulomas with histiocytes, giant cells, and foamy macrophages are seen.4 The reactive infiltrate of EI is more mixed than in SPTL, with small lymphocytes, plasma cells, and eosinophils. Leukocytoclastic vasculitis and extravascular caseous or fibrinoid necrosis also may be present.4,5 Substantial caseous necrosis may extend to the dermis and epidermis with EI. Importantly, EI lacks true tuberculoid granulomas and stains negative for acid-fast bacilli, as it is a reactive rather than a local infectious process, but a history of M tuberculosis exposure is common.4 α1-Antitrypsin deficiency panniculitis results from a deficiency of proteinase activity and can be distinguished from SPTL by a neutrophil-rich panniculitis (Figure 5) as well as the classic appearance of splaying of neutrophils between collagen bundles in the deep reticular dermis. Additionally, the panniculitis is characterized by focal areas of necrotic lobules and septa with an infiltrate of neutrophils and macrophages that abut areas of normal-appearing subcutaneous fat without infiltrate.6 Clinically, the A1ATDP lesions may have ulceration and express an oily substance from fat necrosis. Panniculitis with A1ATDP may precede liver and lung disease.4 Panniculitis from bacterial or fungal infection is more common in immunocompromised patients but should be considered when subcutaneous inflammation and/or necrosis is present. Depending on the responsible organism and the status of a patient’s immune system, infectious panniculitis can have variable presentations, including suppurative granulomas with mycobacterial organisms, a dermal focus of infection if the primary source is cutaneous, or a deeper reticular and subcuticular focus in the subcutaneous fat if the infectious panniculitis occurs from hematogenous spread.4 An example of an infectious panniculitis having more of a granulomatous pattern secondary to Cryptococcus can be seen in Figure 6. Ultimately, special stains to identify infectious organisms (eg, Gram, periodic acid–Schiff, Ziehl-Neelsen) can be ordered to aid in the diagnosis if a responsible organism is not visible on hematoxylin and eosin staining.

Pretibial myxedema is a cutaneous mucinosis associated with thyroid dysfunction. The differential diagnosis includes nephrogenic systemic fibrosis...
Hailey-Hailey disease typically presents as suprabasal blisters with a perivascular and interstitial lymphocytic infiltrate. The differential...

Giant cell tumors of soft tissue present as multilobulated masses in the skin or subcutaneous tissue, most commonly appearing on the trunk, upper...
