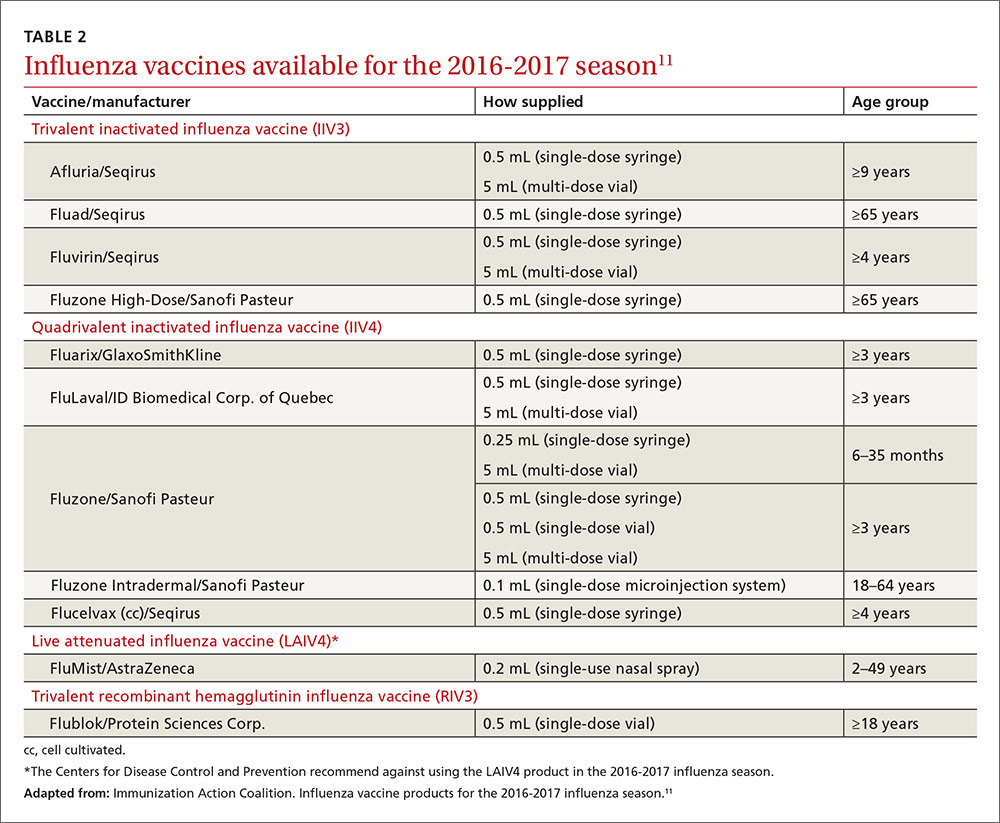
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.
Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.
Antiviral medications for treatment or prevention
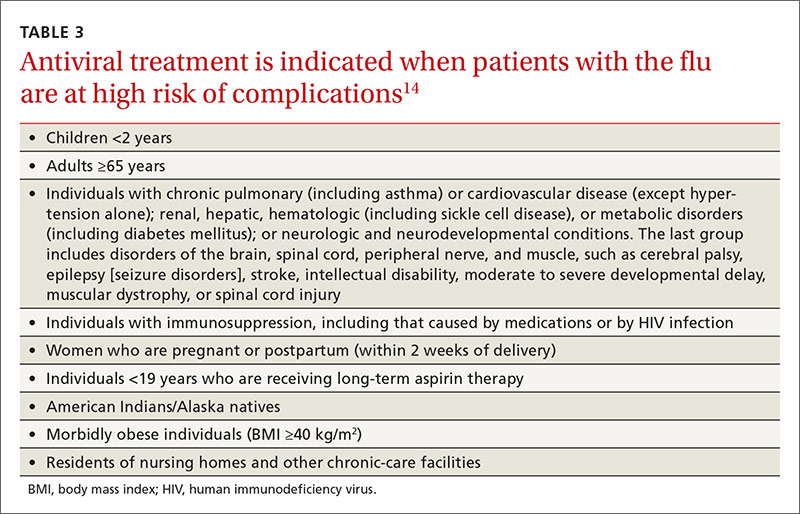
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15