Diagnosis: Gram-negative foot intertrigo
Plain x-rays and computed tomography imaging did not reveal evidence of abscess formation, subcutaneous gas/air formation, or osteomyelitis. Laboratory testing was normal. The patient was empirically started on cefepime, clindamycin, and ketoconazole. Wound therapy, consisting of normal saline washes, use of xeroform gauze to cover the wound, and frequent absorbent dressing changes, was also initiated. Wound cultures were obtained, which later grew Pseudomonas aeruginosa and Enterococcus faecalis. (P aeruginosa was the predominant organism.) We made the diagnosis of gram-negative foot intertrigo.
First described in 1973, researchers have found that P aeruginosa, as well as E faecalis and Staphylococcus aureus, are commonly associated with tinea pedis1 and toe web intertrigo.2 The infection usually involves the lateral 3 toe webs, and can present with malodorous discharge, itchy maceration, edema, and erythema of the surrounding tissue. Non-purulent lower extremity cellulitis is typically caused by beta-hemolytic streptococci residing in the toe webs, and is usually treated empirically with cephalexin or similar antibiotics.3 The presentation of foot intertrigo varies along a spectrum that includes tinea pedis, superinfected maceration, and infectious eczematoid dermatitis, all of which can encourage bacterial superinfections.
In this patient, it is likely that sweating (and consequent skin maceration), tinea pedis, and perhaps even friction between the affected area and the patient’s shoes led to skin ulceration. This then became colonized and infected with bacteria, including gram-negative varieties, which sometimes harbor at the edges of ulcerations. Addressing each of these factors is important to heal the infection.
Differential diagnosis includes other bacterial skin infections
The differential diagnosis includes erysipelas, cellulitis, lipodermatosclerosis, venous eczema, and burns. Erysipelas affects the superficial dermis with well demarcated borders, while cellulitis involves the subcutaneous fat.4 Both are more likely to be caused by beta-hemolytic streptococci, but at first may be difficult to differentiate from similar appearing gram-negative skin/soft tissue infection without microbiologic data.
Patients with lipodermatosclerosis and venous eczema often have a history of chronic venous insufficiency. Lipodermatosclerosis often presents with a subcutaneous panniculitis and hyperpigmentation; venous eczema is often associated with scaling of the involved areas. Although burns can become secondarily superinfected with bacteria, they can be differentiated from a primary bacterial infection by the history or presentation.
Gram-negative infections (especially those caused by Pseudomonas species) should be suspected if a toe web infection does not respond to empiric antimicrobial therapy with first- or second-generation cephalosporins, as their spectrum of antimicrobial activity does not include P aeruginosa. If the infection is severe, it may impact ambulation.2 Predisposing factors for gram-negative toe web infections include obesity, diabetes, moist environments, tight interdigital spaces, and recurrent tinea pedis.4
Administer appropriate wound care and start antimicrobial therapy
Foot intertrigo provides an easy portal of entry for pathogenic organisms.5 Therefore, it is important to modify risk factors from the outset to help prevent superinfection (as occurred with this patient) and other complications. Aggressive treatment of tinea pedis and use of compression stockings to reduce lymphedema are vital for prevention of recurrent infections.3
Physicians should be aware of likely, as well as unlikely, causative pathogens; “typical” skin and soft tissue infections that do not resolve may be due to atypical or gram-negative organisms, and typical first-line antibiotics will do nothing to eradicate them. Antimicrobial susceptibilities and a bacterial culture will steer you to the appropriate antimicrobial therapy. Debridement of the edge of the ulceration is necessary to remove any lingering bacteria.6 And appropriate wound care is paramount and should include the use of techniques to keep the affected areas dry, such as the use of astringent powders along with avoidance of damp shoes and socks.
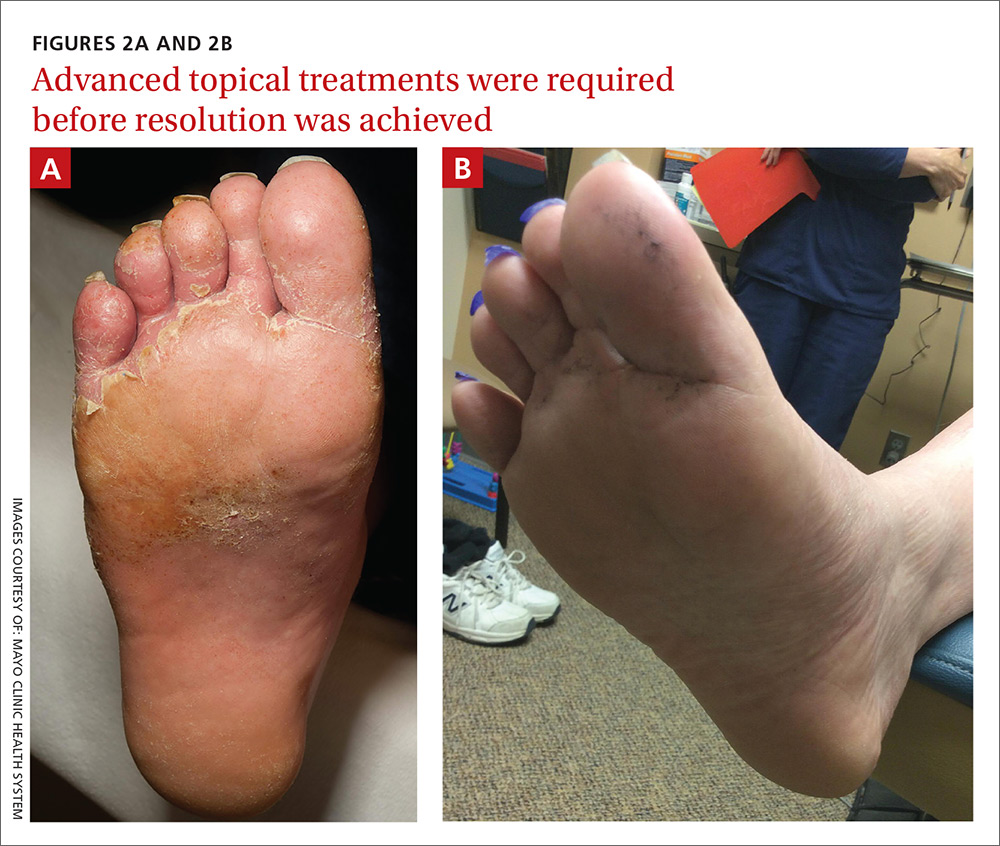
Our patient was switched from empiric therapy to culture-specific IV therapy with vancomycin 2 g every 12 hours, ciprofloxacin 400 mg every 12 hours, and fluconazole 400 mg daily. Two weeks later, he was discharged from the hospital on topical antifungals, oral fluconazole, and daily acetic acid soaks. He did, however, require further advanced topical treatments (miconazole 2% twice daily) due to recurrent flare-ups before complete resolution was achieved 6 weeks after presentation (FIGURES 2A AND 2B).
CORRESPONDENCE
Alberto Marcelin, MD, Department of Family Medicine, Mayo Clinic Health System, 1000 1st Dr NW, Austin, MN 55912; marcelin.alberto@mayo.edu.