CHICAGO –
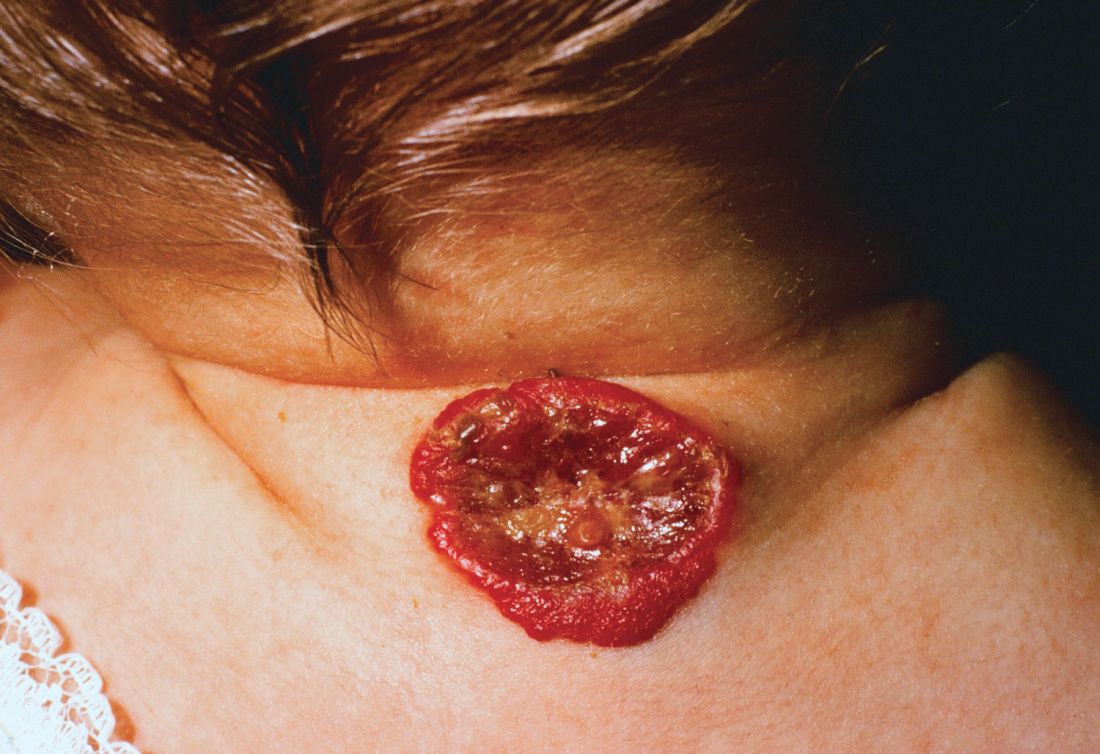
About 16% of infantile hemangiomas become ulcerated at some point during their proliferative phase, said Kate Puttgen, MD, during a talk at the World Congress of Pediatric Dermatology.
Hemangiomas are more likely to ulcerate if they are large, superficial, or segmented; if they’re located in an intertriginous area or are on the mucosa; or if they are of a mixed subtype, said Dr. Puttgen. Patient factors that may increase risk for ulceration include prematurity, low birth weight, and being female.One clinical clue to picking up an infantile hemangioma (IH) that’s destined to ulcerate is an early grayish to white discoloration of the lesion, said Dr. Puttgen, chief of the division of pediatric dermatology at Johns Hopkins Medicine, Baltimore.
“Multimodal therapy is an absolute necessity” in treating an ulcerated IH, said Dr. Puttgen. Using an “all hands on deck” approach – a combination of topical and systemic modalities – can help bring the lesion under control.
Beta-blockers are first-line therapy to manage complicated IHs, with propranolol yielding a 98% response rate for all complicated IHs in the literature, said Dr. Puttgen.
Propranolol can decrease the volume and color of IHs and speed involution, in part by its ability to continue working after the proliferative growth phase of an IH. It’s also been shown to reduce the need for surgery in nasal IH, and it’s well tolerated, she added.
Evidence-based therapies for ulcerated hemangiomas include systemic propranolol at 1-3 mg/kg per day. That protocol will result in a healed ulcer within 2-6 weeks in most of the published case series, Dr. Puttgen noted.
Topical timolol also has evidence supporting its use for an ulcerated IH, and it has been found generally safe. In one study of 30 patients with IH, she said, three had mild adverse events consisting of sleep disturbance, diarrhea, and acrocyanosis. Another study reported success when brimonidine 0.2% and timolol 0.5% were used together. It’s possible, said Dr. Puttgen, that there’s a synergistic effect when combining the selective alpha-2 adrenergic agonist effect of brimonidine with timolol, which provides nonselective beta adrenergic blockade. However, she said, there has been an isolated report of brimonidine toxicity.
The ulcerated IHs need wound care, Dr. Puttgen added, with barrier creams and dressings. Pain management should be considered, because an ulcerated IH may have a large, friable, bleeding area. Pulsed-dye laser can also be a useful treatment modality for an ulcerating IH.
Going beyond the treatments for which the evidence is strongest and moving into more “state-of-the-art” treatments, “there may be a niche role for oral corticosteroids” as combination systemic therapy with propranolol, Dr. Puttgen said.
She shared images from a recently published report, in which she’s the senior author, showing the progression of an ulcerated IH. The hemangioma had received wound care and pulsed-dye laser treatment, and the infant was started on systemic propranolol. After 2 weeks, the IH had decreased significantly in volume, but the ulcerated area had actually increased. With the addition of oral corticosteroids, there was a reduction in ulceration after 2 weeks; and after 5 weeks of prednisolone, “the ulceration resolved without rebound,” said Dr. Puttgen. The corticosteroid was then tapered and propranolol was continued for an additional 2 months, then tapered. By 10 months, the IH had almost completely resolved (Br J Dermatol. 2017 Apr;176[4]:1064-7).
If a corticosteroid is added to propranolol, there may be benefit to a slower propranolol dose, Dr. Puttgen said. She suggests an altered dosing schedule, beginning with 1 mg/kg per day in two or three divided doses. Then, over a period of 2-7 days, the total daily dose can be increased to 1.5 mg/kg per day. Bumping the dose up to 2 mg/kg per day or higher should not happen until after 2 weeks at the reduced dosing schedule, she explained.
Dr. Puttgen disclosed that she is on the advisory board and has received honoraria from Pierre Fabre Dermatologie.
On Twitter @karioakes