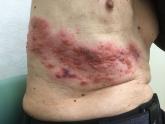
Herpes zoster subunit vaccine (Shingrix) was preferentially recommended over zoster vaccine live (Zostavax) for preventing herpes zoster and related complications Oct. 25 at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Eight committee members voted for the recommendation, and seven voted against it.
After the decision, ACIP Chair Nancy Bennett, MD, professor of medicine at the University of Rochester (N.Y.), noted that it was the “closest vote” in her term as chair of the committee, which provides advice and recommendations on vaccine-preventable diseases to the CDC.In discussions leading up to the vote, some committee members cited potential supply issues, as well as the need for longer-term safety data, among other issues.
“I think it would be nice to see data on a larger population that is not just research-based, especially because we have very little data on ethnic minorities,” said Laura E. Riley, MD, of Harvard Medical School, Boston, who voted against the recommendation.
The vote comes several days after GlaxoSmithKline announced the Food and Drug Administration approval of Shingrix for the prevention of herpes zoster (shingles) in adults aged 50 years or older. In pooled clinical trial results, the vaccine demonstrated greater than 90% efficacy in all age groups, according to a company statement.
Shingrix is a non-live, recombinant subunit vaccine that is given in two doses, intramuscularly. Zostavax, also indicated in individuals aged 50 years or older, is a live attenuated virus vaccine.
In a related decision, ACIP voted 14-1 to recommend Shingrix for prevention of herpes zoster and related complications for immunocompetent adults aged 50 years and older.
They also voted 12-3 to recommend Shingrix to prevent herpes zoster and its complications for immunocompetent adults who previously received Zostavax.