EVIDENCE SUMMARY
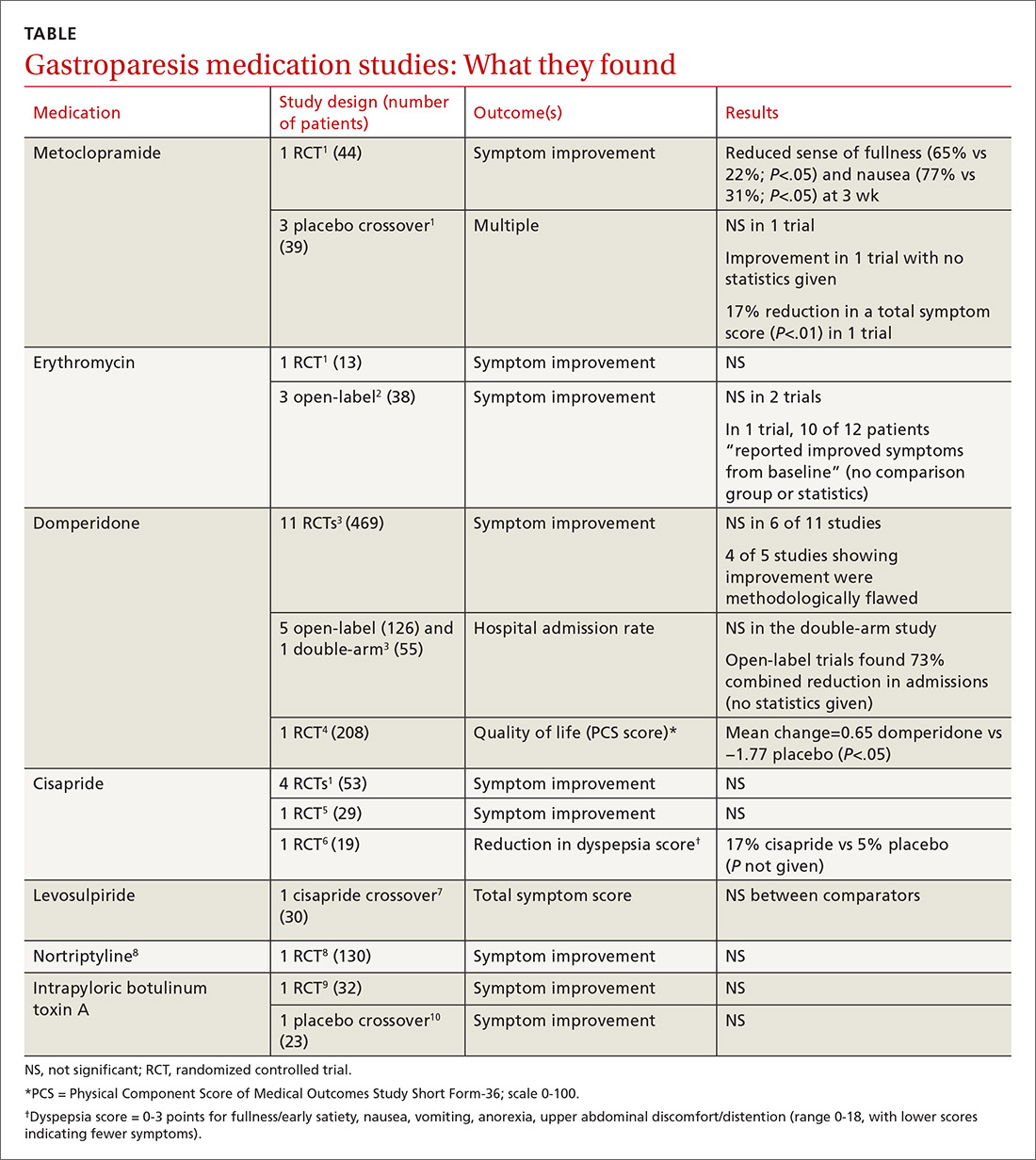
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride