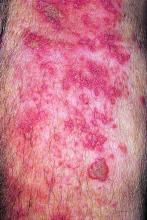
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.