A 1-hour treatment with a low concentration of nitrous oxide, commonly known as “laughing gas,” appears to relieve symptoms of treatment-resistant major depression (TRMD), with effects lasting as long as several weeks, new research suggests.
In a trial with a crossover design, investigators randomly assigned 28 patients with severe TRMD to receive a single 1-hour inhalation of placebo or nitrous oxide once a month over a 3-month period. Participants received an inhalation of placebo; a 25% concentration of nitrous oxide; and a 50% concentration of nitrous oxide. Sessions were conducted 4 weeks apart.
Both doses of nitrous oxide were associated with substantial improvement in depressive symptoms for roughly 85% of participants. However, the 25% concentration had a lower risk for adverse effects, which included sedation, nausea, and mild dissociation, compared to the 50% concentration.
“Twenty-five percent nitrous oxide has similar efficacy, compared to 50% nitrous oxide, and reduced side effects fourfold,” lead author Peter Nagele, MD, MSc, chair and professor of anesthesia and critical care, University of Chicago, said in an interview.
“We also observed that many patients had a 2-week improvement of depressive symptoms after a nitrous oxide treatment,” said Dr. Nagele, who also is professor of psychiatry and behavioral neuroscience.
The study was published online June 9 in Science Translational Medicine.
Further refinement
A previous proof-of-principle study conducted by the same researchers demonstrated that a 1-hour inhalation of 50% nitrous oxide had rapid antidepressant effects for patients with TRMD.
The current phase 2 trial “is a follow-up study to our earlier 2015 pilot trial and was designed to further refine the dose of nitrous oxide needed for antidepressant efficacy,” Dr. Nagele said.
“An important secondary aim [of the current study] was to determine whether a lower dose – 25% – would reduce side effects, and a third aim was to determine how long the antidepressants effects last,” he explained.
To investigate, the researchers enrolled 28 patients (median [interquartile range (IQR)] age 39 years [26-68 years]; 71% women; 96% White) to have three inhalation sessions (placebo, 25%, and 50% nitrous oxide) at 4-week intervals. Twenty patients completed all three inhalation sessions, and four completed ≥1 treatment.
Participants had “sustained and refractory depressive illness,” with a mean illness lifetime duration of 17.5 years and an extensive history of antidepressant drug failure (median, 4.5 [2-10] adequate-dose/duration antidepressants).
Some patients had undergone vagus nerve stimulation, electroconvulsive therapy, or repetitive transcranial magnetic stimulation, or had received ketamine (4%, 8%, 13%, and 8%, respectively).
The primary outcome was improvement on the 21-item Hamilton Depression rating Scale (HDRS-21) score over a 2-week observation period.
‘Stronger evidence’
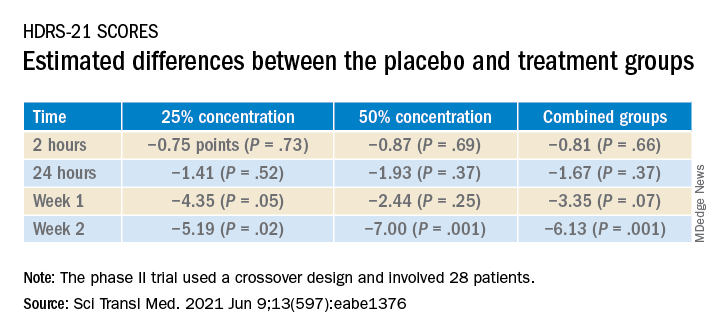
Compared to placebo, nitrous oxide significantly improved depressive symptoms (P = .01). There was no significant difference between the 25% and the 50% concentrations (P = .58).
The estimated difference in HDRS-21 scores between the placebo and various treatment groups are shown in the following table.
To ensure there where were carryover effects between the two doses, the researchers performed an analysis to ascertain whether order of receipt of the higher dose was related to the 2-week HDRS-21 score; they found no significant effect of trial order (P = .22).
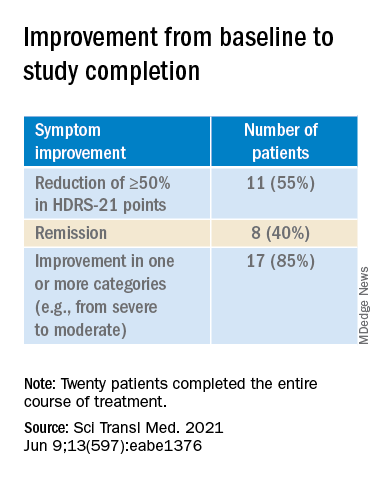
The 20 patients who completed the entire course of treatment “experienced a clinically significant improvement in depressive symptoms from a median baseline HDRS-21 score of 20.5 (IQR, 19.0 to 25.5) to 8.5 (IQR, 2.0 to 16.0) at study completion, corresponding to a median change of −11.0 points (IQR, −3.3 to −14.0 points; P < .0001) after the 3-month study period,” the investigators noted.
The types of treatment response and improvement in depressive symptoms from baseline to study completion are listed in the table below.
There were statistically significant differences in adverse events between the two treatment doses; 47 events occurred following inhalation of the 50% concentration, compared to 11 after inhalation of the 25% concentration. There were six adverse events after inhalation of placebo (P < .0001).
“None of the adverse events were serious, and nearly all occurred either during or immediately after the treatment session and resolved within several hours,” the authors reported.
“We need to be remindful that – despite the exciting results of the study – the study was small and cannot be considered definitive evidence; as such, it is too early to advocate for the use of nitrous oxide in everyday clinical practice,” Dr. Nagele said.
Nevertheless, on the basis of the current findings, he stated.